Issue:October 2023
FORMULATION FORUM - CUBOSOMES – The Next Generation of Lipid Nanoparticles for Drug Delivery
INTRODUCTION
Cubosomes are lipid-based highly stable nanoparticles.1 They are soft structured membrane assemblies of lipid bi-continuous cubic liquid crystalline phases with interior water channels stabilized by polymeric outer corona surfaces. These mesomorphic cubic phases are composed of unique low-melting lipids with a single bilayer bearing continuous membrane lattice structure with pores leading to water channels.2 With the invent of modern new characterization techniques, such as small-angle x-ray scattering, for instance, it has shed light into microstructures and mechanisms of drug delivery of these LNPs. With unique structures, cubosomes provide a significantly higher surface area for their greater abilities to solubilize hydrophobic and hydrophilic molecules with relatively higher loading as opposed to liposomes. These lipid nanoparticles, or cubosomes, can be engineered with highly selective lipid components to deliver a wide range of molecules.3
Ascendia Pharma recently introduced LipidSol®, a lipid-based platform technology for the delivery of small molecules with poor solubility, peptides, gene therapy, and biologics in lipid nanoparticles.4 One of the assemblies could adapt the bi-continuous cubic phases (or cubosomes) depending upon lipid composition and type, which markedly differ from traditional liposomes, in which the outer surfaces are stabilized by polymers or co-polymers with PEG moieties. Cubosomes possess an ability to tune the membrane curvature with larger surface areas independently as opposed to bilayer membranes. In addition, the hydrophobic volume fraction of a monoolein cubosomes is about 100 nm in diameter, which is typically around three times larger than a small unilamellar vesicle (SUV) with similar size.5 As we continue to explore the LipidSol-enabling technologies in drug delivery, the following will shed light on the design and formation of cubosomes with special focus on their applications for delivery of hydrophobic and hydrophilic small and large molecules, including oncology drugs and polynucleotides (DNA, mRNA, and siRNA).
STRUCTURE OF CUBOSOMES
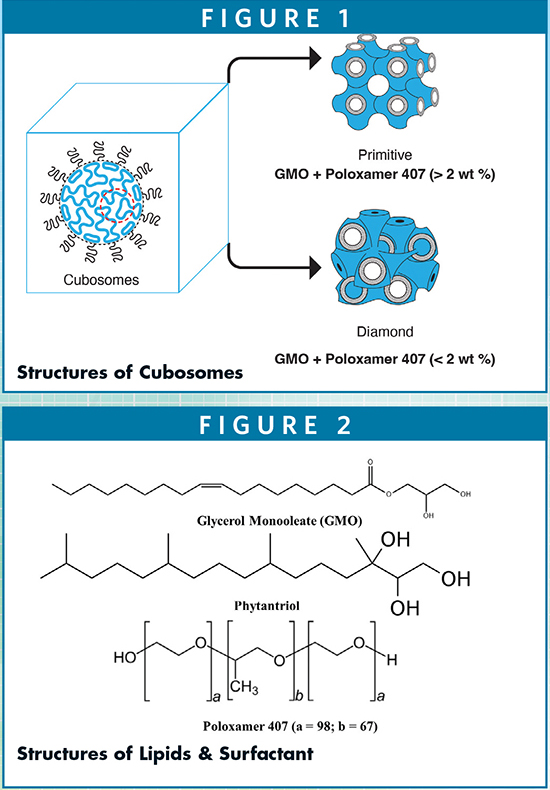
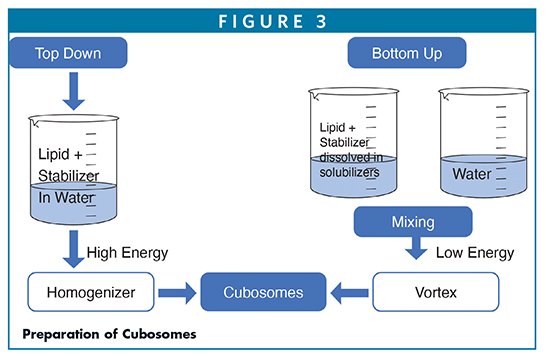
Cubosomes are categorized into two morphological structures, namely primitive cubosomes and diamond based on lipid compositions and types (Figure 1).6 Cubosomes are composed of amphiphilic low-melting monoolein (a monoglyceride) and/or phytantriol and stabilized by polymeric solubilizers/surfactants. As shown in Figure 1, the lipids are typically monoolein or phytantriol, and poloxamer 407 is used as a stabilizer. Their chemical structurers are shown in Figure 2. Upon hydration with water, these lipids spontaneously form cubic liquid crystalline phases arranged in primitive or double diamond assemblies with the phase transition between 43°C and 80°C. The stabilizers inevitably play a crucial role in providing longer stability, controlling the phase boundaries and drug release through the restrictive water channels.
PREPARATION & CHARACTERIZATION OF CUBOSOMES
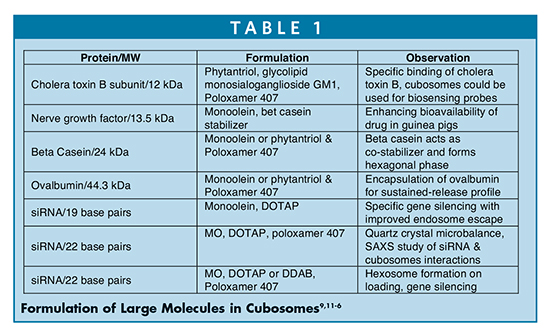
Cubosomes are prepared by the top down (high shear energy) or bottom up (low shear energy) approach as outlined in Figure 3.
It requires the dispersion of lipids in aqueous solution followed by sonication or low-shear mixing or high-shear homogenization. If using organic solvents as hydrotropes, following evaporation, the dried film is hydrated with buffer or saline and sonicated, which results in formation of cubosomes.7 If heating is controlled, there is a lesser chance of degradation of lipids and formation of homogeneous dispersions. To alleviate the rise in temperature during sonication, a temperature-controlled cup horn system is utilized as an alternative to sonicator tip. Spicer, et al investigated stable monoolein-derived cubosomes with lower polydispersity via sonication of an aqueous solution containing ethanol as a hydrotrope.8 This process can further be optimized using propylene glycol and polyglycerol ester to avoid any inherent residual solvents like ethanol for delivery of proteins and vaccines as described by Rizwan, et al.9 Other methods, such as microfluidics, are also applied to further offset the heating issues and enhance the production at much larger scale.10
DSC, NMR, and fluorescence spectroscopy techniques have been used for characterization of lipid vesicle morphologies and bilayer structures, but a high-energy small angle x-ray scattering (SAXRD) method is commonly used to identify the cubosomes.2 The characteristic diffraction patterns in SAXRD from the fingerprints and spacing in between the rings identifies the certain morphology of the lipid packing to determine the packing of these lipids. Furthermore, cryo-transmission electron microscopy (Cryo-TEM) is also an excellent technique for characterization of the lipids in the cubosomes. Cubosomes, for example, designed with glycerol monooleate, polyglycerol ester, and poloxamer 407 show the continuous structures with vesicle formation at the surface with water channel surrounding the exterior. Dynamic light scattering (DLS) is used to measure the particle size distribution and polydispersity.
APPLICATION OF CUBOSOMES
Large Molecules
Cubosomes can encapsulate small and large molecules, including proteins. Tabel 1 shows the encapsulation of proteins and polynucleotides in the cubosomes with increasing molecular weight order.
Small Molecules
In cancer therapeutics, for example, monoolein cubosomes entrapped with doxorubicin showed an increased rate of release at lower pH. Other examples include release of folic acid in cubosomes composed of monoolein stabilized with poloxamer 407 for targeting cancer cells. Another study involves the subcutaneous administration of 5-fluouracil (5-FU) in monoolein cubosomes.17
Table 2 shows encapsulation of small molecule oncology drugs in cubosomes composed of monoolein and Poloxamer 407 used as a stabilizer.
Table 3 shows the cubosomes in ocular delivery of drugs composed of monoolein (GMO) and Poloxamer 407 as stabilizer.
Whilst the list of drugs encapsulated in cubosomes is exhaustive, environmental factors, such as pH, temperature, and pressure, could play an important role in drug release. Other factors include the hydrophobic and hydrophilic nature of molecules and size of interstitial hydrophobic cargo space. For example, release of lipophilic drugs, such as griseofulvin, diazepam, and propofol among others, from monoolein cubosomes is dependent upon the partition coefficient and burst mechanism that results in an immediate release within 20 mins. This can be fine turned by incorporating with pH-sensitive lipids and stabilizers to yield the sustained-release profile.23 Cubosomes derived from phytantriol suspension encapsulated with a poorly soluble drug cinnarizine can lead to controlled-release profile over 50 hours as opposed to 5 hours from monoolein suspensions.24 Ionic polymers incorporated in cubosomes will have direct impact on release profile of the drugs in a controlled manner. For instance, poloxamer 407 stabilized cubosomes derived from monoolein with a grafted copolymer showed an extended release at neutral and acidic pH.25 Cubosomes derived from monoolein and Poloxamer 407 and stabilized with polyvinyl alcohol can lead to controlled release of an anti-inflammatory drug etodolac at lower dosages compared to orally administered tablets.26 In other examples, tetrandrine-encapsulated cubosomes composed of monoolein stabilized with poloxamer 407 showed enhanced transcorneal permeation opposed to free drug.27
CAPABILITIES IN LIPID NANOPARTICLES
As we continue to explore new molecules with enabling innovative technologies like LipidSol, we find limitless opportunities to find the solutions for those chemical entities, making the insoluble soluble. Ascendia remains at the forefront of developing formulations of those practically insoluble and/or less bioavailable molecules and can help bring them to the advanced stages of clinics. With our expertise in formulation development built with state-of-the art GMP manufacturing capabilities, we can help innovators interested to move forward with Phase 1 and Phase 2 clinical studies. We can find the solutions quickly by employing our enabling platform technologies for formulating small and large molecules, proteins, and biologics in LNPs and cubosomes.
CONCLUSION
As we continue to explore the nanotechnologies in delivery of drugs by “making the insoluble soluble,” we find cubosomes to be the most attractive lipid nanoparticles (LNPs) of all entities as they are derived from biodegradable, simple low-melting chain lipids and can be easily scaled up with high drug loading and stabilized with PEG-ylated polymers for longer shelf-life, and for efficient and sustained delivery of potent small and large molecules. Our expertise in top down and bottom formulation approaches, as outlined in Figure 2, can lead to smarter design and delivery of innovative molecules across all modalities. As we embark on expanding our state-of-the art cGMP manufacturing facilities for sterile drug products, we expect more new molecules will be evaluated in LNPs, especially at least some preferably utilizing cubosomes in the future.
REFERENCES
- H. Umar, H. A. Wahab, A. M. Gazzali, H. Tahir and W. Ahamd, Cubosomes: Design, development and tumor targeted drug delivery applications, Polymer, 2022, 14, 3118.
- H. M. G. Barriga, M. N. Holme, and M. M. Stevens, Cubosomes; the next generation of smart lipid nanoparticles?, Angew. Chem. Int. Ed. Eng., 2019, 58, 2958-2978.
- B. Angelov, A. Angelova, M. Drechsle, V. M. Garamus, R. Mutafchieva, and S. Lesieur, Identification of large channels in cationic PEGylated cubosome nanoparticles by synchrotron radiation SAXS and Cryo-TEM imaging, Soft Matter. 2015; 11:3686–3692.
- J, Huang and S. Ali, LIPIDSOL®: The Liposomes – Chemistry, Properties and Applications of Lipid Nanoparticles, Drug Dev. Delivery, 2023.
- V. Meli, C. Caltagirone, A. M. Falchi, S. T. Hyde, V. Lippolis, M. Monduzzi, M. Obiols-Rabasa, A. Rosa, J. Schmidt, Y. Talmon Y, and S. Murgia, Docetaxel-loaded fluorescent liquid-crystalline nanoparticles for cancer theranostics, Langmuir. 2015; 31:9566–9575.
- R. Tenchov, R. Bird, A. E. Curtze and Q. Zhou, Lipid Nanoparticles – from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement, ACS Nano, 2021, 15, 16982-17015.
- Nakano M, Sugita A, Matsuoka H, et al. Small angle x–ray scattering and 13C NMR investigation on the internal structure of cubosomes, Langmuir. 2001, 17, 3917–3922.
- P. T. Spicer, Progress in liquid crystalline dispersions: Cubosomes, Curr. Opinion Colloid Interface Sci. 2005, 10, 274-279.
- (a) S. B. Rizwan, D. Assmus, A. Boehnke, T. Hanley, B. J. Boyd, T. Rades, S. Hook, Preparation of phytantriol cubosomes by solvent precursor dilution for the delivery of protein vaccines, Eur. J Pharm. Biopharm. 2011, 79, 15-22; (b) S. B. Rizwan, W. T. McBurney, K. Young, T. Hanley, B. J. Boyd, T. Rades and S. Hook, Cubosomes containing the adjuvants imiquimod and monophosphoryl lipid A stimulate robust cellular and humoral immune responses, J. Cont. Release, 2013, 165, 16-21.
- S. P. Akhlaghi, I. R. Ribeiro, B. J. Boyd and W. Loh, Impact of preparation method and variables on the internal structure, morphology, and presence of liposomes in phytantriol-Pluronic(®) F127 cubosomes, Colloids Surfaces B Biointerfaces. 2016, 145, 845-853.
- S. J. Fraser, X. Mulet, L. Martin, S. Praporski, A. Mechler, P. G. Hartley, A. Polyzos, and F. Separovic, Surface immobilization of bio-functionalized cubosomes: Sensing of proteins by quartz crystal microbalance, Langmuir, 2012, 28, 620-627.
- X. Che, Z. Wang, Y. Liu , Y. Sun, and H. Liu, Sustained release of nerve growth factor from highly homogenous cubosomes stabilized by β-casein with enhanced bioactivity and bioavailability, RSC Adv. 2016, 6, 114676-114684.
- J. Zhai, L. J. Waddington, T. J. Wooster, M-I. Aguilar, and B. J. Boyd, Revisiting β-casein as a stabilizer for lipid liquid crystalline nanostructured particles, Langmuir. 2011; 27:14757-14766.
- C. Leal, N. F. Bouxsein, K. K. Ewert, and C. R. Safinya. Highly efficient gene silencing activity of siRNA embedded in a nanostructured gyroid cubic lipid matrix, J. Am. Chem. Soc. 2010, 132, 16841-47.
- B. Tajik-Ahmadabad, A. Mechler, B. W. Muir, K. McLean, T. M. Hinton, F. Separovic and A. Polyzos, Chem. Bio. Chem. 2017; 18:921-930.
- G. Zhen, T. M. Hinton, B. W. Muir, S. Shi, M. Tizard, K. M. McLean, P. G. Hartley, and P. Gunatillake, Glycerol monooleate-based nanocarriers for siRNA delivery in vitro, Mol. Pharm. 2012; 9:2450-2457.
- X. Jin, Z. H. Zhang, S. L. Li, E. Sun, X. B. Tan, J. Song, and X. B. Jia, A nanostructured liquid crystalline formulation of 20 (S)-protopanaxadiol with improved oral absorption. A nanostructured liquid crystalline formulation of 20(S) protopanaxadiol with improved oral absorption, Fitoterapia, 2013. 84: p. 64-71.
- D. Bei, T. Zhang, J. B. Murowchick and B. C. Youan, Formulation of dacarbazine-loaded cubosomes. Part III. Physicochemical characterization. AAPS PharmSciTech, 2010, 11, 1243-1249.
- M. R. Cheng, Q. Li, T. Wan, B. He, J. Han, H. X. Chen, F. X. Yang, W. Wang, H. Z. Xu, T. Ye, and B. B. Zha, Galactosylated chitosan/5-fluorouracil nanoparticles inhibit mouse hepatic cancer growth and its side effects. World journal of gastroenterology: WJG, 2012. 18(42): p. 6076.
- L. Gan, S. Han, J. Shen, J. Zhu, C. Zhu, X. Zhang, and Y. Gan, Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: improving preocular retention and ocular bioavailability. Int. J. Pharm. 2010, 396,179-187.
- J. Huang, T. Peng, Y. Li, Z. Zhan, Y. Zeng, Y. Huang, X. Pan, C. Y. Wu, and C. Wu, Ocular cubosome drug delivery system for timolol maleate: preparation, characterization, cytotoxicity, ex vivo, and in vivo evaluation, AAPS PharmSciTech, 2017, 18, 2919-2926.
- Y. Chen, Y. Lu, Y. Zhong, Q. Wang, W. Wu, and S Gao, Ocular delivery of cyclosporine A based on glyceryl monooleate/poloxamer 407 liquid crystalline nanoparticles: preparation, characterization, in vitro corneal penetration and ocular irritation. J. Drug Target., 2012, 20, 856-863.
- B. J. Boyd, Characterization of drug release from cubosomes using the pressure ultrafiltration method, Int. J. Pharm. 2003, 260, 239-247.
- T. H. Nguyen, C. J. H. Porter, I. Larson, and B. J. Boyd, Phytantriol and glyceryl monooleate cubic liquid crystalline phases as sustained-release oral drug delivery systems for poorly water soluble drugs I. Phase behaviour in physiologically-relevant media, J. Pharm. Pharmacol. 2010, 62, 856-865.
- M. Kluzek, A. Tyler, S. Wang, R. Chen, C. M. Marques, F. Thalmann, J. M. Seddon, and M. Schmutz, Influence of a pH-sensitive polymer on the structure of monoolein cubosomes, Soft Matter. 2017, 13, 7571-7577.
- S. Salah, A. A. Mahmoud, and A. O. Kamel, Etodolac transdermal cubosomes for the treatment of rheumatoid arthritis: ex vivo permeation and in vivo pharmacokinetic studies, Drug Deliv. 2017, 24, 846-856.
- R. Liu, S. Wang, S. Fang, J. Wang, J. Chen, X. Huang, X. He X, and C. Liu, Liquid crystalline nanoparticles as an ophthalmic delivery system for tetrandrine: Development, characterization, and in vitro and in vivo evaluation, Nanoscale Res Lett. 2016, 11, 254.

Jim Huang, PhD
Founder & CEO
Ascendia Pharmaceuticals
j.huang@ascendiapharma.com
www.ascendiapharma.com
Dr. Jim Huang is the Founder and CEO of Ascendia Pharmaceuticals, Inc. he earned his PhD in Pharmaceutics from the University of the Sciences in Philadelphia (formerly Philadelphia College of Pharmacy and Sciences) under Joseph B. Schwartz. He has more than 20 years of pharmaceutical experience in preclinical and clinical formulation development, manufacturing, and commercialization of oral and parenteral dosage forms. His research interests are centered on solubility/bioavailability improvement and controlled delivery of poorly water-soluble drugs through nano-based technologies.

Shauket Ali, PhD
Sr. Director, Scientific Affairs & Technical Marketing
Ascendia Pharmaceuticals
shaukat.ali@ascendiapharma.com
www.ascendiapharma.com
Dr. Shaukat Ali joins Ascendia Pharmaceuticals Inc. as Senior Director of Scientific Affairs and Technical Marketing after having worked in the pharma industry for many years. His areas of expertise include lipid chemistry, liposomes, lipid nanoparticles, surfactant-based drug delivery systems, SEDDS/SMEDDS, oral and parenteral, topical and transdermal drug delivery, immediate- and controlled-release formulations. He earned his PhD in Organic Chemistry from the City University of New York and carried out his post-doctoral research in Physical Biochemistry at the University of Minnesota and Cornell University. He has published extensively in scientific journals and is inventor/co-inventor of several US and European patents.
Total Page Views: 6685















