Issue:May 2021
FORMULATION FORUM - Considerations in Formulation Development of Injectable Solutions
INTRODUCTION
Injectable solutions are intended for administration to the human body by intravenous, intramuscular, or subcutaneous injection. Advantages of injectables include fast onset, and other parent routes of administration, reproducible PK/efficacy profile, high bioavailability as a result of bypassing the oral absorption barrier, and suitability of administration under hospital setting. In order to meet the safety, efficacy, and quality standards of parenteral dosage forms, injectable solutions have to be sterile, low pyrogen, and meet the requirements of compendia specifications, such as >90% label claim, related substance level lower than the tox qualified level, content uniformity, pH, osmolality, particulate matter, essentially free of visual foreign matter, etc.
The main challenges of parenteral dosage forms are achievement of formulation stability, compatibility of drug substance with packaging components, and sufficient drug concentration within a reasonable pH range and without using excipient levels that causes blood incompatibility and tissue irritation issues. Those requirements necessitate a comprehensive characterization of drug
The process of parenteral formulation development can be divided into preformulation studies, prototype formulation development, accelerated stability studies, packaging selection, process development to evaluate critical process parameters, scale-up, manufacturing of demonstration and GMP baches, and their long-term stability studies for up to 24-36 months. substance physiochemically. In addition, the critical manufacturing processing conditions of injectable liquids have to be evaluated and stringently controlled in order to meet requirements for assay/related substance, CU, leachable/extractables, sterility, pyrogen, and particulate matter specifications
PREFORMULATION
Preformulation studies are critical for injectable solution development, which covers the measurement of solubility/stability as a function of pH, pKa, partition, moisture sorption/desorption, polymorphism and crystallinity by differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and powder X-ray diffraction, salt form screening, stability of drug substance, assessing the compatibility of API and excipients using stress testing, and forced degradation to evaluate the effect of pH, temperature, humidity, light, and oxidizing agents. In addition, compound solubility in commonly used solvent, cosolvent, surfactant, solubilizer, and complex agents will be useful in setting the direction of formulation development.
The aqueous solubility of a compound is dependent on its state of ionization, including the ratio of ionized to unionized fraction. The degree of ionization can be estimated using the Henderson–Hasselbach equation. For weak acidic compounds, (HA) is:
pH = pKa + log[A–]/[HA];
for weakly basic compounds, (BH) is:
pH = pKa + log[B]/[BH+],
where Ka is the ionization constant of the dissociation constant.
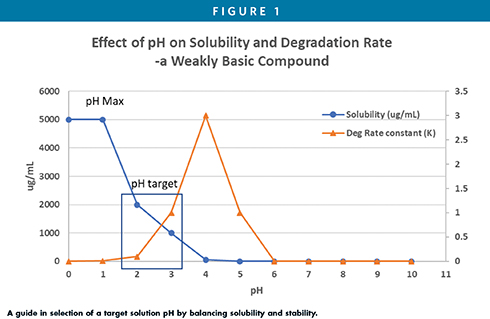
Effect of pH on API solubility and stability is most important for solution formulation development of ionizable compounds, which will guide the selection of formulation pH in the solution dosage form (Figure 1). Salt or co-crystal form is another factor that may also affect the compound solution solubility; salt form screen or in-situ salt formation should be evaluated to find a salt form that enhances compound solubility.
FORMULATION DEVELOPMENT
Prototype formulation development covers selection of buffer, pH, stabilizers, isotonicity agents, antioxidants, preservatives, and solubility and/or viscosity-enhancing agents.
pH & Buffer Selection
The pH is one of the critical aspects of parenteral formulation, which should have a target pH as much as possible close to physiological pH. An ionizable compound can be solubilized to the desired concentration by pH adjustment. The acceptable range is pH 2–11 for intravenous and intramuscular injection and pH 4-9 for subcutaneously injection due to potential irritation issue. The solution pH can be controlled by a buffer, such as acetate, phosphate, citrate, histidine, TRIS, and others, to an ideal range of pH 5-8. The buffer capacity should be kept to a minimum to enable body fluid to adjust the formulation pH quickly to physiological pH; otherwise, an irritation at the injection site may be present. For IV infusion, the solution pH is typically controlled by the salt form of the drug or by acids and bases without buffer due to in vivo tolerability considerations. In some cases, a compromise may be required between solubility and stability of the drug substance in order to find a target formulation pH in which both drug loading and product stability are acceptable (Figure 1).
Osmolarity
Parenteral formulations should be ideally isotonic whenever possible. Dextrose, Mannitol, glycerol, and sodium chloride are the most commonly used agents to adjust the tonicity of a solution formulation. Hypertonic solutions will cause blood cell deformation, whereas a hypotonic solution will lead to rupture of blood cell that results in hemolysis. Formulations with an osmotic value in the range of 250 to 350 mOsm are acceptable; any exception to this range should be justified case by case. For example, for formulation containing solvents, such as ethanol, even though the measured osmolality is high for those formulations, because the small solute can permeate through blood cell membranes rapidly to reach equilibrium inside and outside of the cell membrane, high osmolality value may not proportionally impact on tonicity in vivo.
Solubilizers
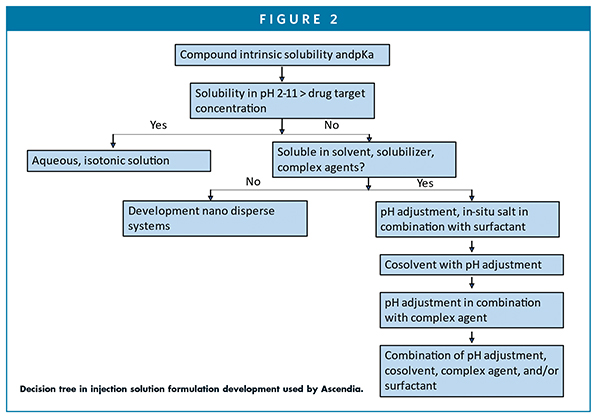
Due to the high percentage of insoluble compounds in the development stage, solubilization becomes an important task for pharmaceutical scientists in their daily works. In addition to alteration of pH and in situ salt formation, solubilizers, such as surfactant, solvent, co-solvent, complexing agents, or their combinations, are routinely utilized to solubilize poorly water-soluble compounds. Co-solvents are used when a drug substance has insufficient solubility in a simple aqueous or pH-adjusted vehicle. The water-soluble organic solvents and surfactants used in marketed injectables include propylene glycol, polyethylene glycol 300/400, glycerin, ethanol, polysorbate 80, Cremophor EL, N-methyl-2-pyrrolidone (NMP), etc. These solvents are usually used in combinations with each other with an aid of pH adjustment. A decision tree can be used as a guide for the injectable solution development (Figure 2).
Container & Closure
Compatibility of injectable solution with stopper and container is another critical aspect for injectable formulation development. For high solvent/surfactant content formulations, assessment of potential discoloring, deformation, and leachable of the stopper as a result of incompatibility should be evaluated as soon as the prototype formulation is selected. For early formulations, a Teflon- coated stopper is usually used to shorten the development timeline to avoid any potential compatibility issues. Leaching of alkaline materials from glass may cause an increase in solution pH, which could be significant for un-buffered formulations, wherein the compound’s solubility and stability are sensitive to the pH change. Container-closure integrity should be evaluated for each injectable solution product during development prior to manufacturing and during stability study to ensure integrity of injectable products is maintained.
PROCESS DEVELOPMENT & SCALE-UP
Process development is centered around three of the most critical areas for sterile products: sterility, stability, and particulate matter. Parenteral manufacturing requires filling and sealing to be carried out in a Class 100 environment for injectables processed under aseptic conditions, the manufacturing process of which should be validated using media runs to ensure sterility of the finished dosage forms. For terminal sterilized products, they can be manufactured in Class 10,000 environment, which however, requires sterilization cycle validation that ensures 6-log reduction of bioburden. Critical process parameters that affect product potency and stability, such as light, bulk holding, filter compatibility, tubing selection, nitrogen protection, etc should also be evaluated during process development and scale up.
Compatibility With Infusion Sets
IV formulations are typically injected by syringe and administered using infusion sets. Evaluations should be undertaken to evaluate if the compound is absorbed into the syringe and infusion set. Studies should show that no significant losses occur following filtration, injection, or infusion under the conditions of administration.
Plasma & Blood Compatibility
Compounds to be administered intravenously must be compatible with plasma and blood. Evaluation of the compounds in lead formulations should be undertaken initially in the blood and plasma of relevant preclinical species.
Plasma precipitation may result in embolism and subsequent death. A plasma precipitation test should be conducted to reduce the risk of embolism during in vivo studies. The study can be conducted by diluting the formulation with control plasma and observe for signs of precipitation. If precipitation is evident, an alternative formulation should be sought to avoid the precipitation or the concentration of the compound in the formulation should be lowered to prevent plasma precipitation.
The compound in the formulation should not cause hemolysis, significant crenellation, or erythrocyte clumping. There should also be no adverse reactions, such as thrombophlebitis, observed at the injection site following IV administration. A further evaluation in human blood and plasma should be undertaken in the formulation of choice for clinical trials.
Formulation Stability
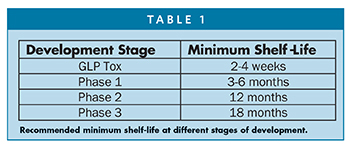
A minimum storage life of 18 months, preferably at room temperature, is desired for a commercial parenteral solution formulation. If sufficient solution stability cannot be obtained by storing at room temperature, refrigerated storage at 2°C -8°C or at -20oC may be considered. Solution stability studies should be undertaken to predict storage life under both conditions. It is expected that an ideal injectable solution will demonstrate suitable stability in solution over the target storage life period. However, when adequate solution stability cannot be obtained, the development of a lyophilized product should be considered. The recommended minimum shelf-life is different at various stages of development. Table 1 summarizes the minimum recommended shelf-life at different stage of development.
SUMMARY
Injectable solutions offer an attractive alternative to oral dosage form due to fast onset, reproducible PK/efficacy profile, high bioavailability as a result of bypassing the oral absorption barrier, and suitability of administration under hospital setting. The main challenges of parenteral dosage forms are achievement of formulation stability, solubility, sterility, and reduction of blood incompatibility and tissue irritation issues. Formulation development of injectable solution can be divided into preformulation studies, prototype formulation development, accelerated stability studies, packaging selection, process development to evaluate critical process parameters, scale-up, manufacturing of demonstration and GMP baches, and long-term stability studies.

Jim Huang, PhD
Founder & CEO
Ascendia Pharmaceuticals
j.huang@ascendiapharma.com
www.ascendiapharma.com
Total Page Views: 40142










