Issue:June 2020
FORMULATION FORUM - Application of Captisol® Technology to Enable the Formulation of Remdesivir in Treating COVID-19
INTRODUCTION
The numbers are startling for the outbreak of a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). Coronavirus disease (COVID-19) is affecting more than 227 countries and territories. Globally, total cases are nearly 5.9 million as of May 291 and total deaths surpassed 363,000.1 In the US, there are more than 1.74 million confirmed total cases with more than 102,000 deaths. Also, the cumulative hospitalization rate in the US is approximately 73 per 100,000 people; and in the 65+ age group, this rate is more than triple.2
On May 1, 2020, Gilead Sciences and the US Government announced an Emergency Use Authorization (EUA) for remdesivir3, the first antiviral therapeutic for treating COVID-19. A week later, the Japanese Regulatory Authority approved VEKLURY® (remdesivir).4 Japan, United Kingdom, Taiwan, and the US are currently the only countries to authorize use of remdesivir for treatment of COVID-19. The European Medicines Agency is continuing its rolling review of remdesivir, and on May 11, 2020, recommended expanding the compassionate use of the investigational medicine remdesivir so that more patients with severe COVID 19 can be treated.5
Gilead announced it will donate its entire remdesivir stockpile to the US Government (~1.5 million doses) and boost production to provide more than 1.5 million additional treatment courses by year end.6 If required, production is expected to continue to ramp up through 2021 to replenish and grow the stockpile (Figure 1). Ongoing clinical trials continue to evaluate remdesivir’s safety and efficacy.
To meet the high demand for remdesivir is an immense task and requires extraordinary coordination and cooperation on an unprecedented and global scale. Gilead said it will build a geographically diverse consortium of pharmaceutical and chemical manufacturing companies, and announced signing nonexclusive licensing pacts with five generic drug makers, voluntarily sharing its knowledge to produce remdesivir across the globe. Gilead recognized early that to deliver remdesivir worldwide to the patients in need requires establishing numerous partnerships.7,8
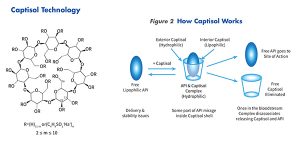
Captisol is a proprietary sulfobutylether-cyclodextrin process-specific composition that is a mixture of regional and positional isomers consistently produced via a patented all-aqueous process originally discovered at the University of Kansas.9 The motivation for Captisol’s discovery was to provide a parenterally safe material broadly applicable across all drug classes for solubilizing and stabilizing intractable drug candidates as opposed to historically used toxic cosolvents and surfactants. It works predominantly by a transient and reversible cyclodextrin (CD) host-guest association complex whereby a hydrophobic region or moiety of a wide range of substrates (drugs) is attracted to the hydrophobic cyclodextrin cavity (Figure 2). This forms the basis for use to increase solubility, stability, and bioavailability of drugs.10 Captisol can also have extra cavity interactions with the 4-carbon linker or electrostatic with the anionic sulfonate. And more recently, it has been observed that other types of complexes, such as non-inclusion complexes can form, and CDs can self-assemble to form nanosized aggregates; both can contribute to their solubilizing properties.11
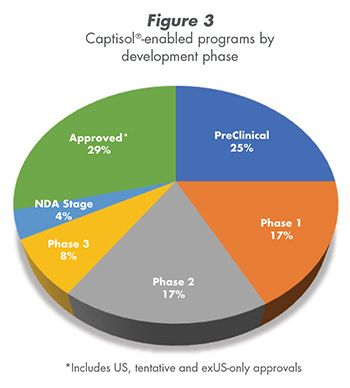
Fifteen human drug products have been approved using Captisol (including this EUA, tentative approvals, and approvals outside the US). There are many Captisol®-enabled products in all phases of product development (Figure 3).
As the owner of the innovator technology Captisol, Ligand Pharmaceuticals has invested to increase cGMP production capacity, establishing manufacturing at two geographically distinct locations, establishing storage and distribution from multiple sites in the US and overseas, and also establishing relationships with several Contract Research Organizations (CROs) to practice monograph test methods to perform release testing for Captisol partners.
Ligand has also established vast Drug Master Files in the US, Canada, China, and Japan, and has a voluminous safety database grown by Ligand and partners investing in preclinical development. Ligand has invested in continuous improvement in the control and cGMP manufacturing of Captisol. Ligand and its manufacturing partners have discovered all-aqueous processes to reduce or remove undesirable impurities such as phosphate, chloride, and color that have resulted in proprietary compositions.
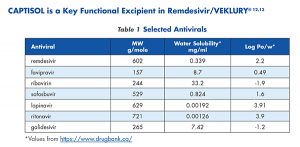
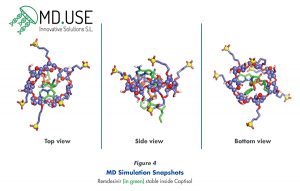
Remdesivir, like some other antivirals listed in Table 1, has poor predicted solubility14 and poor stability.15 Molecular Dynamics (MD) was used to perform a preliminary view of the interaction of Captisol with remdesivir (Figure 4).16 Several sets of MD simulations were performed starting from pre-assembled Captisol-remdesivir complexes in pre-equilibrated-explicit Simple Point-Charge (SPC) water model. Six representative structures of Captisol, using different locations for 6 or 7 sulfobutylether substitutions in the positions 2, 3, and 6 of βCD were employed. Four replicas of each system (50 ns-long each) were performed, using different orientations of remdesivir inside the cavity. For additional technical details for the MD simulations, please see Garrido et.al.17 or contact Dr. Garcia-Fandiño.
The interactions of Captisol with remdesivir readily produce a clear solution (Figure 5) or lyophilized solid presentations of remdesivir, i.e. Lyophilized Powder and Injection Solution with drug – Captisol amounts in Table 2 as described in the EUA Fact Sheet for Health Care Providers12 and Gilead Remdesivir Pharmacy Guide.13
As a ready-made solution, the EUA Fact Sheet and Pharmacy Guide states to store remdesivir injection solution (contains 6 grams Captisol) at refrigerated temperature, whereas for the lyophilized powder (contains 3 grams Captisol), store at room temperature below 30°C.12,13
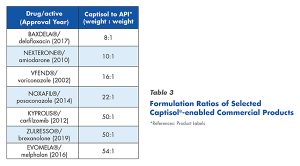
The use of high Captisol to API ratios in Captisol®-enabled products is not unusual (Table 3), but does dictate bulk requirements. Given the large amounts of Captisol used in the formulations, quality, purity, and reproducibility are all very critical.
SUMMARY
Ligand supports Gilead and the remdesivir manufacturing consortium with Captisol. More Captisol than ever is required to meet Gilead’s bold goals of making remdesivir available to COVID-19 patients in the US and to hundreds of countries around the world. Ligand announced it plans investment in capital equipment that may possibly allow the cGMP annual production capacity for Captisol to increase to as much as 500 MT. Captisol production is supported by other raw material suppliers. For example, Wacker Chemie AG supplies native β-cyclodextrin that undergoes further processing by Ligand. Critical links throughout the Ligand supply chain are being maintained and managed to expand the supply of Captisol. Ligand, along with a network of suppliers and manufacturers, is among the critical supply chain links to support Gilead and its partners to urgently produce remdesivir.
REFERENCES
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)”. ArcGIS. Johns Hopkins University. Accessed May 29, 2020.
- Hospitalization Rate, https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html. Accessed May 29, 2020.
- Food and Drug Administration (FDA) issues an Emergency Use Authorization (EUA) for emergency use of remdesivir for the treatment of hospitalized 2019 coronavirus disease (COVID-19) patient, EUA Letter, https://www.fda.gov/media/137564/download. Accessed May 1, 2020.
- Gilead Announces Approval of Veklury® (remdesivir) in Japan for Patients with Severe COVID-19, https://www.gilead.com/news-and-press/press-room/press-releases/2020/5/gilead-announces-approval-of-veklury-remdesivir-in-japan-for-patients-with-severe-covid19. Accessed May 7, 2020.
- EMA recommends expanding remdesivir compassionate use to patients not on mechanical ventilation, https://www.ema.europa.eu/en/news/ema-recommends-expanding-remdesivir-compassionate-use-patients-not-mechanical-ventilation. AccessedMay 11, 2020.
- Working to Supply Remdesivir for COVID-19, Remdesivir Manufacturing Projections, https://www.gilead.com/purpose/advancing-global-health/covid-19/working-to-supply-remdesivir-for-covid-19.
- Voluntary Licensing Agreements for Remdesivir, https://www.gilead.com/purpose/advancing-global-health/covid-19/voluntary-licensing-agreements-for-remdesivir.
- Gilead partners with 5 generic drug makers on remdesivir supply expansion, May 12, 2020. https://www.pharmamanufacturing.com/industrynews/2020/gilead-partners-with-5-generic-drugmakers-on-remdesivir-supply-expansion/.
- V.J. Stella, R.A. Rajewski, Sulfobutylether-_-Cyclodextrin, International Journal of Pharmaceutics (2020), https://doi.org/10.1016/j.ijpharm.2020.119396. Available online May 4, 2020.
- Loftsson, M. Másson, and M.E. Brewster, Self-association of cyclodextrins and cyclodextrin complexes, (2004) J. Pharm Sci. 93(5),1091-9.
- Messner, S.V. Kurkov, P. Jansook and T. Loftsson, Self-assembled cyclodextrin aggregates and nanoparticles, International Journal of Pharmaceutics (2009), DOI: 10.1016/j.ijpharm.2009.11.035, Epub, Dec 4, 2009.
- Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of Remdesivir (GS-5734), https://www.fda.gov/media/137566/download.
- Pharmacy Guide, https://www.remdesivir.com/us/downloads/remdesivir_pharmacy_guide.pdf.
- DRUG BANK, predicted solubility and Log P by ALOGPS, http://www.vcclab.org/lab/alogps/.
- Nate Larson, Compositions comprising an rna polymerase inhibitor and cyclodextrin for treating viral infections, US20190083525A1, https://patents.google.com/patent/US20190083525A1/en, March 21, 2019.
- MDUSE, http://mduse.com/en/.
- Garrido, P.; Calvelo, M.; Garcia-Fandiño, R.; Piñeiro, Á. Rings, Hexagons, Petals, and Dipolar Moment Sink-Sources: The Fanciful Behavior of Water around Cyclodextrin Complexes, Biomolecules 2020, 10, 431, https://www.mdpi.com/2218-273X/10/3/431, March 10, 2020.

James Pipkin, PhD, Vice President, New Product Development
Dr. Pipkin joined Ligand in 2011, following Ligand’s acquisition of CyDex Pharmaceuticals. He joined Cydex in 2001. Dr. Pipkin supports Captisol partners and directs internal development of new applications, and products utilizing Captisol, whether applied to new molecular entities (NMEs), or reformulations of existing drugs via traditional and the 505(b)(2) regulatory pathways. Prior to joining the Company, he was Executive Director for CMC Services at Oread Laboratories, Research Fellow with Merck Research Laboratories in the INTERx Research Division and West Point PR&D facilities where he was involved in the design and evaluation of controlled release devices for ophthalmic and oral delivery, and he was at The Squibb Institute for Medical Research where he directed preformulation activities. He has contributed to numerous presentations, monographs/chapters, publications and patents and holds an MS and PhD in Pharmaceutical Chemistry from The University of Kansas.

Vince Antle, PhD, Senior Vice President, Technical Operations
Dr. Antle joined Ligand in 2011 following Ligand’s acquisition of CyDex Pharmaceuticals. He joined CyDex in 2005. Dr. Antle is currently responsible for quality assurance, internal drug product quality, operations, distribution and logistics for Captisol. From 1999 to 2005, Dr. Antle was Technical Operations Manager and Head of Process Development at EaglePicher Pharmaceuticals Services. Prior to 1999, Vince was the Group Leader for the Combinatorial Chemistry Department of MDS Panlabs in Bothell Washington. Dr. Antle has contributed to publications, presentations, and patents and holds a PhD from the University of Cincinnati in Medicinal Chemistry, and a BA in Chemistry from the University of Minnesota, Morris.

Rebeca García Fandiño, PhD
Rebeca Garcia-Fandino is a computational chemistry researcher working at the Center for Research in Biological Chemistry and Molecular Materials (CiQUS), at Santiago de Compostela University (Spain). She is one of the main co-founders and CEO of MD.USE Innovations. She is author of more than 30 research articles on Molecular Dynamics and Quantum methods applied to different systems, from small reactive molecules to supramolecular systems, including cyclodextrins. She is also author of several book chapters, a patent and software register. The aim of her research is the design of new and improved antibacterial/antineoplasic agents based on understanding their action mechanism, both in solution and at the level of membranes using computational tools. She can be reached at rebeca.garcia.fandino@usc.es.
Total Page Views: 12897







![Remdesivir Compositions [EUA FACT SHEET FOR HCPS12 and Pharmacy Guide13]](https://drug-dev.com/wp-content/uploads/2020/06/T2-300x69.jpg)