Issue:November/December 2020
FORMULATION FORUM - Age-Appropriate Pediatric Formulation Development
BACKGROUND
Pediatric patients are defined as patients aged from birth to less than 16 or 18 years, depending on the country. The EU guideline on clinical investigation of medicinal products in the pediatric population (CPMP/ICH/ 2711/99) uses the following age groups in relation to developmental stages.
- Preterm newborn infants
- Term newborn infants (0-27 days)
- Infants and toddlers (1 month to 23 months)
- Children (2-11 years)
- Adolescents (12-16 or 18 years)
It has been recognized that the requirements of drug formulation for children are different from those of adults. The lack of appropriate pediatric formulations is the main obstacle for use of many drugs in children. Historically, children have been treated with off-label used medications by means of extemporaneous compounding, which, however, is not ideal due to potential safety/efficacy concerns. As a result of the US Best Pharmaceuticals for Children Act (BPCA) and Pediatric Research Equity Act (PREA) of 2007 and European Union medicinal products for pediatric use in 2006, more and more companies are encouraged to develop and generate clinical data for the pediatric population in order to gain accelerated drug approval and extended market exclusivity.
The development of pediatric formulations can be challenging due to specific requirements of the patient population. A pediatric formulation should consider the following factors: difference in physiological and pharmacokinetic of patient populations, dosage form selection, route of administration, dose accuracy, dose flexibility, drug and excipient tolerability (safety and toxicity), patient compliance (palatability/ swallowability), stability, and drug accessibility.
BIOPHARMACEUTICAL CONSIDERATIONS & CLINICAL DEVELOPMENT STRATEGY
One of the most important issues in the development of medicinal products for pediatric patients is selection of the most appropriate formulation in relation to patient age. Due to substantial changes in the absorption, distribution, metabolism, and excretion (ADME) profile in pediatric populations, particularly during the first years of life, there is a significant variability in the pharmacokinetics of the same drug and dosage form between the pediatric and adult populations. For example, due to the immaturity of the pediatric population, the intestinal mucosa of infants and young children is more permeable than that of adults; as a result, there is significant increased absorption for the pediatric population than that in the adults. In addition, the factors impacting drug bioavailability, such as the gastrointestinal pH, gastrointestinal motility, gastric emptying time, and intestinal transport systems, is different in children from those in adults.
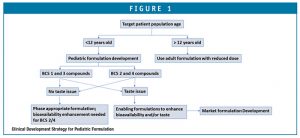
A decision tree in clinical formulation strategy, based on drug BCS classification, age, and drug palatability, is illustrated in Figure 1 as a guide for pediatric formulation development. A phase-appropriate enabling formulation strategy can be adapted if the compound is BCS class 1 or 3 and does not present issues in palatability. However, for a BCS 2 and 4 compound with palatability issues, development of a market image/commercial formulation is desired to avoid biopharmaceutical risk in BA/BE between clinical and market formulations. Typically, initial studies of a pediatric formulation is done in an adult population to demonstrate acceptable BABE; once the safety and efficacy of the formulation in adults is demonstrated, clinical trials in children can then be conducted using the pediatric formulations.
DOSAGE FORM SELECTION
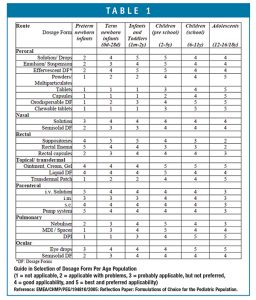
One of the most important issues in the development of medicinal products for pediatric patients is its appropriateness for the target patient age without presenting problems in palatability and swallowability. According to the EU guideline on the clinical investigation of medicinal products in the pediatric population (CHMP/ICH/2711/99), a matrix combining different age groups, routes of administration, and dosage forms has been developed to assist in dosage form selection (Table 1). The age classification has been further divided into pre-school children (2-5 years) and school children (6-12 years) because of the significant changes in ability to handle some dosage forms between 2-12 years of age.
EXCIPIENT SAFETY CONSIDERATIONS
In addition to the incompatibility of excipients with API that generate toxic impurity, the interactions of certain excipients under intracellular environments may also produce toxic metabolites that may interfere with children’s development processes. It is critical that the daily update amount of excipients in a pediatric formulation be within the allowable daily Intake of the excipients and be within the limit of previously approved pediatric products in the major pharma markets, such as the US and EU.
Although excipients are generally considered to be inert, there are cases that excipients may play a role in enhancing solubility and bioavailability and thus impact on drug safety and efficacy. Particularly for neonates and infants, because they have immature metabolic systems, the drug itself or toxic metabolites may accumulate inside the body that cause side effects. The excipients associated with potential toxicity in pediatric oral, topical, and intravenous formulations are: propylene glycol used as solvent, which may cause central nervous system (CNS) effects, especially in neonates and children under 4 years; ethanol as solvent may introduce intoxication due to its ease to cross the BBB; benzyl alcohol as solvent and preservative have “Gasping syndrome” in neonates; benzoic acid as preservative may cause jaundice in neonates; parabens used as preservative might generate oestrogenic and potential reproductive effects; sorbitol as a sweetener causes GI discomfort as a result of osmotic diarrhoea; saccharin as sweetener and dyes as coloring agents may induce hypersensitivity and photosensitivity reactions, etc. Those excipients should be used with caution in very young patients.
Overall, the following aspects are to be considered when selecting excipients for use in pediatric formulations: 1) the function of the excipient; 2) the safety profile of the excipient for children in the target age group(s) on the basis of single and daily exposure; 3) the expected duration of the treatment (short term or long term); 4) the severity of the condition to be treated (risk/benefit ratio); 5) the patient acceptability (palatability/swallowability), etc.
FORMULATION CONSIDERATIONS
Ideally, a pediatric formulation should be a flexible, good-tasting, sub-divisible dosage form that is an oral dispersible, oral liquid form, or granules that can be sprinkled to children’s foods or drinks. It is desired that pediatric medicines allow for flexible and precise dosing for children at different developmental stages with swallowing difficulties. Pediatric formulations should also be developed with taste, color, and texture that are acceptable to children of different ages and different cultures. Taste-masking is one of the biggest challenges in pediatric formulation development, which can be achieved by coating the API, complexing the API with excipients, eg, polymers, and by adding sweeteners and flavors to the formulation. Prior to a human taste panel for evaluation of formulation palatability, an e-tone machine can be explored to screen taste-masking formulations.
Most pediatric formulations are liquid dosage forms, including solutions, micron or nano-suspensions, emulsions, syrups, and less frequently, elixirs with a targeted dosing volume of </=5-10 ml for children. Pediatric formulations should ensure palatability and dose accuracy for administration. Liquid solution and suspension formulations are preferred due to advantage in ease of dose adjustments and accurate dose uniformity for infants and younger children. However, the production of liquid formulations may be limited by the solubility and stability of drugs, and the requirements of taste-masking agents, preservatives, and solubility of excipients. The API may need to be micronized or nanosized before incorporated to suspension to increase drug loading and solubility. Furthermore, salt formation or solubilization technologies, such as Ascendia’s nano-technology platforms in nanoparticle engineering, amorphous nano, and nano-emulsions may be explored to improve drug solubility and bioavailability. The recent advances in nanotechnology delivery systems have resulted in the development of nanomedicines for the treatment of various disease conditions. Nanomedicine has advantages over traditional formulations in terms of enhancement of safety and efficacy by increasing solubility and bioavailability of insoluble drugs, optimizing drug loading and stability, enabling taste-masking, reducing local irritation in GI and injection site, and enabling targeted delivery of drugs to specific targets tissues, etc.
From a product quality perspective, residual solvents, heavy metals, potentially genotoxic impurities, degradants from drug/excipient incompatibilities and impurities from excipients should be controlled for pediatric products. In addition, it should be made aware that in some parts of the world, particularly in tropical areas, refrigeration may not be available, and the climates there have high temperatures and humidity levels that may impact on the product’s chemical and physical stability. Child-resistant packaging is required, and dosing devices (droppers, measuring cups, graduated pipettes) should be evaluated as a part of the pediatric product development.
SUMMARY
The lack of appropriate pediatric formulations is the main obstacle for use of many drugs in children. Pediatric formulations make precision, personalized medication for children possible, which could also benefit the drug manufacturers with accelerated drug approval and extended market exclusivity. The development of pediatric formulations can be challenging due to specific requirements of the patient population. A pediatric formulation should consider the following factors: difference in physiological and pharmacokinetic of patient populations, dosage form selection, route of administration, dose accuracy, dose flexibility, drug and excipient tolerability (safety and toxicity), patient compliance (palatability/ swallowability), stability, and drug accessibility. u
To view this issue and all back issues online, please visit www.drug-dev.com.

Jim Huang, PhD
Founder & CEO
Ascendia Pharmaceuticals
j.huang@ascendiapharma.com
www.ascendiapharma.com
Total Page Views: 7229