Issue:January/February 2024
DRUG DEVELOPMENT - Using a Novel Deep Cyclic Inhibition Mechanism to Treat Broad Range of RAS-Mutant Cancers
INTRODUCTION
In recent decades, there has been significant progress in the treatment of a wide range of cancers, with new immune-oncology therapies including programmed cell death protein 1 (PD-1) inhibitors reaching the market and several key regulatory approvals of new targeted therapies including both mitogen-activated protein kinase kinase (MEK) inhibitors and KRAS G12C inhibitors.1-4 Targeted therapies have rapidly advanced to standard-of-care for treatment of many solid tumors, demonstrating clinical efficacy as monotherapies or in combination with other agents.1,3,4 While representing significant advances in care for millions of patients, they also are associated with tolerability issues and drug resistance and are limited to use in specific or small subsets of patients.2,5 Most importantly, they do not address a primary challenge in the treatment of cancer that continues to elude drug developers – the ability to safely target and kill tumor cells while sparing healthy cells.
There remains a substantial need to enhance and improve upon current treatment approaches and to broaden the therapeutic activity of cancer therapies to make them appropriate and effective options for more patients. Next-generation therapies will require novel approaches in drug delivery and targeting, some of which may be counterintuitive to conventional treatment protocols, to be able to safely and effectively address underlying cancer-causing mutations and create better outcomes for more patients.
TARGETING RAS MUTATIONS
In several types of cancer, including pancreatic, melanoma, colorectal, and non-small cell lung cancer, tumors can be driven by mutations of RAS or RAF genes in the mitogen-activated protein kinase (MAPK) pathway.3 Activating mutations of RAS or RAF genes in the MAPK pathway are observed in approximately 30% of all cancer patients, and inappropriate or abnormal activation of this pathway is observed in up to 50% of all tumors and represents one of the most highly used signaling pathways in oncology.6,7 In aggressive solid tumors of the pancreas, skin, lungs, and colon, mutations in RAS or RAF genes are even more common.8 For example, approximately 40% of lung cancers and approximately 90% of pancreatic cancers are due to RAS or RAF genetic mutations.6,8
The most common types of RAS genes – KRAS, HRAS, and NRAS – encode proteins that play an important role in cell signaling.3,6 When RAS genes are mutated, cells grow uncontrollably and evade death signals. RAS mutations also make cells resistant to many available cancer therapies.5 Currently, RAS-selective inhibitors each target single specific RAS mutations that drive different cancer tumors, including the most common KRAS G12C mutation.6 They are also typically designed to sustain target engagement 24/7 to shut down the MAPK pathway chronically.3,5 But treatment based on this chronic inhibition strategy can yield undesirable side effects given that healthy cells also rely on the MAPK pathway, and patients can become resistant to drugs that are specific to a single mutation, as the constant selective pressure against, say, KRAS G12C can cause tumors to mutate again and become reliant on a different mutation in KRAS. Researchers at Immuneering are working to address the question: rather than targeting individual RAS mutations and treating chronically, is it possible to achieve broad therapeutic activity in a way that focuses on malignant cells while minimizing damage to healthy cells?
A NEW APPROACH BASED ON DEEP CYCLIC INHIBITION OF THE MAPK PATHWAY
Immuneering’s journey began with a counterintuitive observation from the company’s proprietary informatics platform. First-generation inhibitors of the MAPK pathway were effective in reversing disease-associated gene expression changes at early time points, such as 3 hours and 6 hours post-administration, but by 24 hours were amplifying disease-associated transcriptomic changes. These observations led the company to conduct extensive research, the results of which support the notion that tumor cells and healthy cells need the MAPK pathway, but in different ways.9 Tumor cells need continuous MAPK pathway signaling to grow and divide, whereas healthy cells can tolerate more moderate or sporadic levels of MAPK signaling. In other words, tumor cells need the MAPK pathway like we need air (a constant supply), and healthy cells need the pathway like we need water (on a more intermittent basis). Thus, healthy cells likely can go several hours without “water,” or MAPK signaling. The company’s observations strongly support further assessment of a unique and counterintuitive approach to patient dosing known as deep cyclic inhibition (DCI).
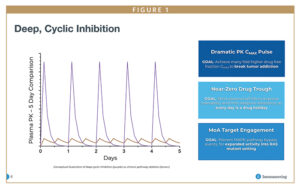
The concept of DCI is to focus therapeutic intervention more against tumor cells than healthy normal cells by deeply cycling and disrupting the MAPK pathway in a rapid series of on and off cycles instead of aiming for 24/7 chronic disruption. This novel approach has two essential goals:
- Hit the tumor hard with pulses of inhibition to break tumor addiction to the MAPK pathway – reaching levels of pharmacokinetic (PK) Cmax many fold higher than traditional “chronic” therapeutic approaches.
- Quickly drop off to a near-zero drug trough or drug level (before the 24-hour mark) to give healthy cells an opportunity to reset and restore homeostatic MAPK pathway signaling – enabled by features including a short therapeutic half-life of approximately 2 hours.
This two-step process is repeated at least once daily in an ongoing cycle. All the action happens behind the scenes based on the drug’s chemical structure, so for patients, it is a very simple matter of taking the drug orally once or twice a day.
Earlier generation cancer therapies are designed to have a long half-life to ensure 24/7 suppression of the MAPK pathway. Designing therapies to have a shorter half-life is essential to support the DCI process – it enables high peak drug exposures (or Cmax) that can then swiftly drop to near-zero drug troughs. Patients essentially experience a daily “drug holiday.” While this may seem counterintuitive compared to currently available treatment approaches, DCI has the potential to target and kill cancer cells more effectively by limiting adaptive resistance, a process where cells upregulate compensatory pathways in response to drug-related activities, while improving drug tolerability.
A very important consideration related to DCI is the potential to broadly target a range of RAS mutations including KRAS, HRAS, and NRAS. This contrasts sharply with most current therapies that target only one specific RAS mutation.3,5,6 The DCI approach targets MEK (a key protein kinase in the MAPK signaling pathway) downstream of RAS and thus could have broader potential applications in treatment of some of the most challenging tumor types.
FIRST-EVER DEMONSTRATION OF DCI IN HUMANS
The DCI approach has been modeled extensively in preclinical research using animal models, proprietary humanized 3D tumor growth assays (3D-TGAs), pharmacogenomics modeling, and in vitro and in vivo models to assess safety and efficacy along with optimal patient populations for treating different RAS-mutant cancers. Researchers at Immuneering leveraged more than 100 tumor models, including 75 different models displaying various RAS mutations.10 Immuneering assessed whether DCI was an optimal therapeutic approach for a range of cancers, including pancreatic, lung, colorectal, melanoma, thyroid, sarcoma, breast, ovary, liver, and neuroblastoma, among others. Targeting this range of tumor models was essential to determine which types of cancer may be addicted to the MAPK pathway and responsive to the DCI mechanism of action. Data from preclinical studies showed the company’s lead product candidate, called IMM-1-104, resulted in comparable to greater tumor growth inhibition versus standards of care and was well tolerated.
Building on this research, Immuneering recently announced the first-ever use of DCI in humans. Positive initial PK, pharmacodynamic (PD), and safety data from a Phase 1/2a clinical trial of IMM-1-104 were presented at the American Association for Cancer Research (AACR) Annual Meeting in April 2023 showing that DCI has potential applications across a number of RAS-mutant solid tumors.11 The Phase 1/2a clinical trial was designed to evaluate the safety, tolerability, PK, PD, and preliminary efficacy of IMM-1-104 in patients with RAS-mutant advanced or metastatic solid tumors, including pancreatic, colorectal, lung, and melanoma. Results from the trial presented at the AACR meeting included the following:
- Significant PK Cmax levels (the plasma concentration of therapy) observed with IMM-1-104 of >2,000 ng/mL (or approximately 1-μM drug free-fraction at 160 mg once daily oral dose).
- >90% PD inhibition of phosphorylated extracellular signal-regulated kinase (pERK) with IMM-1-104 compared to pre-treatment baseline for patients at the third dose level (160 mg once daily oral).
- A median plasma half-life of 1.94 hours observed with IMM-1-104 across the first three dose levels evaluated (40 mg, 80 mg, and 160 mg once daily oral), in patients with pancreatic and colorectal cancer with different RAS mutations, including KRAS-G12D, the most common mutation present in pancreatic cancer.
- IMM-1-104 was well tolerated with no dose limiting toxicities.
Immuneering announced completion of the dose escalation portion of the Phase 1/2a trial of IMM-1-104 in June 2023, achieving this important milestone ahead of the company’s original timeline.12 Additional safety data are now expected in early 2024.
ACCELERATING THE DCI MECHANISM
The company’s second development program IMM-6-415 has also demonstrated preclinically the potential to broadly target MAPK-dependent cancers regardless of the underlying mutation in RAS or RAF. IMM-6-415 is designed to target RAS- and RAF-mutant tumors through DCI of the MAPK pathway, with an accelerated cadence enabled by an exceptionally short therapeutic half-life. While IMM-6-415 has a shorter half-life (about 20 minutes in mice) than IMM-1-104 (about 2 hours in humans) it is still shown to inhibit RAS- and RAF-mutant tumor models. Despite their design differences, the goal of IMM-6-415 and IMM-1-104 is the same – to deprive cancer cells of the continuous pathway signaling they need to rapidly grow and divide while providing healthy cells with the intermittent signaling they need to function.
Promising preclinical data highlighting the therapeutic activity of IMM-6-415 were presented at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2023.13 Researchers at Immuneering assessed IMM-6-415 alone and in combination with encorafenib, a BRAF inhibitor approved for the treatment of melanoma and certain types of colorectal cancer, in a series of preclinical models of RAS- and RAF-mutant disease. Results showed the following:
- >60 humanized 3D-TGA models, including 30 BRAF class I-mutant tumor models, showed a high sensitivity profile for IMM-6-415 in a wide range of MAPK-driven tumor types, including models of RAS- and RAF-mutant disease.
- As monotherapy, IMM-6-415 demonstrated anti-tumor activity in over 50% (34 of 66) of the 3D-TGA models tested, including 30 BRAF mutant preclinical models in which 19 (63%) showed activity.
- Monotherapy treatment with encorafenib or IMM-6-415 displayed superior tumor growth inhibition (TGI) compared to binimetinib in melanoma and colorectal BRAFV600E tumor models.
- IMM-6-415 in combination with encorafenib achieved greater TGI in vivo than encorafenib plus binimetinib in BRAFV600E colorectal cancer and melanoma tumor models. For reference, the combination of encorafenib plus binimetinib was recently approved by the US FDA for the treatment of adults with BRAFV600E metastatic non-small cell lung cancer.14
These data suggest that DCI of the MAPK pathway with an accelerated cadence is active in tumors caused by RAS and RAF mutations and further support the potential of IMM-6-415 as monotherapy or in combination regimens to treat solid tumors. Immuneering anticipates filing an investigational new drug (IND) application for IMM-6-415 in the fourth quarter of 2023.
THE FUTURE OF CANCER TREATMENT
By challenging the conventional 24/7 inhibition paradigm, DCI has the potential to redefine the treatment landscape for RAS- and RAF-mutant cancers. It offers a novel and potentially more effective therapeutic strategy with improved tolerability. As with all new approaches in the treatment of cancer, support must be deeply rooted in data. The DCI mechanism is supported by extensive preclinical and now clinical data demonstrating its potential to be translated on a larger scale.
Immuneering’s progress in understanding the DCI mechanism can also present opportunities to rethink treatment methods in other areas. The approach is not limited to MEK inhibition. It could potentially be applied to additional targets or pathways that: 1) are inappropriately activated in a disease state; 2) demonstrate a biologic addiction to sustained signaling; and 3) allow the DCI cycle to align with underlying disease biology. Immuneering will continue to follow the data to expand and optimize the potential of DCI to play a role in treatment of a broad range of cancers in the years ahead.
REFERENCES
- Cancer Research Institute. (2022, Feb 10). PD-1 / PD-L1 Landscape. https://www.cancerresearch.org/pd-1-pd-l1-landscape.
- Leilei, A. Chen, J., Yan, H., He, Q., Luo, P. Xu, Z., et al. Research Status and Outlook of PD-1/PD-L1 Inhibitors for Cancer Therapy. Drug Des Devel Ther. 2020 Sep 8;14:3625-3649. doi: 10.2147/DDDT.S267433.
- Cheng, Y. and Tian, H. Current Development Status of MEK Inhibitors. Molecules. 2017 Sep 26;22(10):1551. doi: 10.3390/molecules22101551.
- BioSpace. (2022, July 1). MEK Inhibitors Clinical Development Market Approval Insight 2026. https://www.biospace.com/article/mek-inhibitors-clinical-development-market-approval-insight-2026/.
- Kun, E., Tsang, Y.T.M., Ng, C.W., Gershenson, D.M. and Wong, K.K. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat Rev. 2021 Jan;92:102137. doi: 10.1016/j.ctrv.2020.102137. Epub 2020 Dec 16.
- Conroy. M., Cowzer. D., Kolch. W. and Duffy, A.G. Emerging RAS-directed therapies for cancer. Cancer Drug Resist. 2021 Apr 8;4(3):543-558. doi: 10.20517/cdr.2021.07.
- Yaeger, R. and Corcoran, R.B. Targeting alterations in the RAF-MEK pathway. Cancer Discov. 2019 March;9(3):329-341. doi: 10.1158/2159-8290.CD-18-1321.
- Waters, A.M. and Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb Perspect Med. 2018 Sep 4;8(9):a031435. doi: 10.1101/cshperspect.a031435.
- Burotto, M., Chiou, V.L., Lee, J-M. and Kohn, E.C. The MAPK pathways across different malignancies: A new perspective. Cancer. 2014 Nov 15;120(22):3446-56. doi: 10.1002/cncr.28864.
- Immuneering Corporation. (2023, March 5). Immuneering Presents Preclinical Data with Lead Program IMM-1-104 Supporting Universal-RAS Activity. https://immuneering.com/2023/03/05/immuneering-presents-preclinical-data-with-lead-program-imm-1-104-supporting-universal-ras-activity/.
- Immuneering Corporation. (2023, April 18). Immuneering Announces Positive Initial Phase 1 Pharmacokinetic, Pharmacodynamic and Safety Data for IMM-1-104 Universal-RAS Program; Accelerates Study Timeline. https://ir.immuneering.com/news-releases/news-release-details/immuneering-announces-positive-initial-phase-1-pharmacokinetic.
- Immuneering Corporation. Immuneering Completes Dose Escalation in the IMM-1-104 Phase 1 Clinical Trial for RAS-Mutant, Advanced Solid Tumors. (2023, June 5). https://ir.immuneering.com/news-releases/news-release-details/immuneering-completes-dose-escalation-imm-1-104-phase-1-clinical.
- Immuneering Corporation. Immuneering Presents Preclinical Data Demonstrating Encouraging Anti-Tumor Activity for IMM-1-104 and IMM-6-415 at AACR-NCI-EORTC Conference (2023, October 12). https://ir.immuneering.com/news-releases/news-release-details/immuneering-presents-preclinical-data-demonstrating-encouraging.
- Pfizer Inc. U.S. FDA Approves Pfizer’s BRAFTOVI® + MEKTOVI® for BRAF V600E-Mutant Metastatic Non-Small Cell Lung Cancer (2023, October 12). https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-braftovir-mektovir-braf-v600e.

Dr. Ben Zeskind is Co-founder and Chief Executive Officer of Immuneering Corporation, a public, clinical-stage oncology company dedicated to developing medicines for broad populations of cancer patients, and has served in these roles at the company since February 2008. He earned his SB in Electrical Engineering and Computer Science, his PhD in Bioengineering from Massachusetts Institute of Technology (MIT), and his MBA from Harvard Business School, where he was recognized as a Baker Scholar, the highest award for distinction.
Total Page Views: 3072