Issue:March 2022
DRUG DEVELOPMENT - The Promise of Cutting-Edge Microbiome-Based Therapeutics
INTRODUCTION
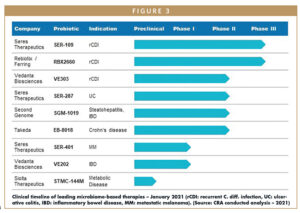
In recent years, there has been increasing evidence that imbalances in bacteria living in the human microbiome (dysbiosis) contribute to a host of diseases that can be effectively treated by restoring harmonious bacterial populations (symbiosis).1 This connection has established manipulation of the microbiome as a unique therapeutic approach and inspired research and development of microbiome-based therapies. Currently, there are several microbiome-based therapies progressing through various stages of clinical development and commercial readiness, with probiotic therapies being the most advanced. Several manufacturers of probiotic therapies recently announced positive topline results from pivotal trials assessing these therapeutics for treatment of C. difficile infection (CDI), and researchers from the University of Birmingham in the UK recently published results from a new study adding to a growing body of evidence showing that fecal microbiota transplantation (FMT) is highly successful in treating patients with CDI.2
As novel associations between the human microbiome, health, and disease continually emerge, and therapies including probiotics advance in clinical development, this progress shows the high growth potential of the microbiome therapeutics market.3,4 Yet, barriers remain in development and commercialization, including (a) the inability to clearly demonstrate the clinical applications of microbiome-based therapies, (b) unclear guidelines for their use in physicians’ treatment algorithms, and (c) the nascency of the field.5,6 Developers must strategically address and overcome these challenges to successfully drive development and eventual adoption of microbiome-based therapies.
DISEASES ASSOCIATED WITH MICROBIOTA DYSBIOSIS
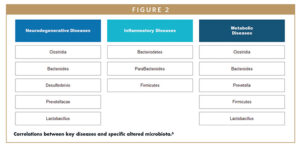
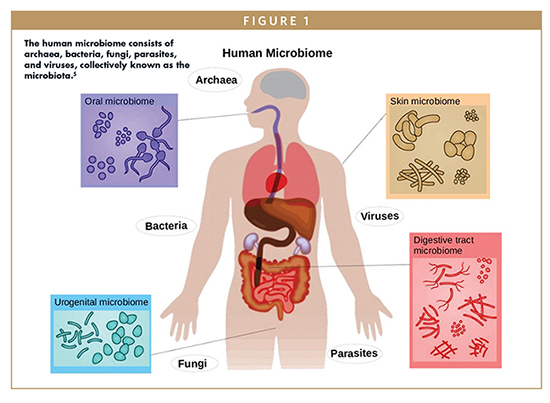
The microbiome is the diverse community of all microorganisms, helpful and harmful, in the human body. These communities of microorganisms exist in the skin, nasopharynx, oral cavity, respiratory tract, gastrointestinal tract (GI), and female reproduction tract.1 Everyone’s microbiome composition is different – genetics, environmental influences, diet, and health all subtly alter the composition of the microbiota. Clinical research around microbiome-based therapeutics focuses on manipulating bacteria in the gut, which is the most abundant and diverse microbial community in humans. Imbalances in the microbiota have recently been identified as the direct cause of more than 25 diseases and conditions, with significant alterations in the composition of gut microbiota shown to be the cause of neurodegenerative, inflammatory, and metabolic diseases.6
Neurodegenerative Diseases: The gut microbiome and brain communicate in a bidirectional manner via the gut-brain axis. The microbiome’s modulation of the brain and spinal cord (central nervous system) is mediated by microbial chemical signals (called metabolites), which can influence neuroendocrine function, the level of circulating neurotransmitters in the body, and behavioral changes. In addition, psychiatric and neurologic disorders can lead to the reduction of microbiome diversity and GI-related symptoms. Studies have shown that dysbiosis is associated with some neurodegenerative diseases, such as Parkinson’s disease, and a range of psychiatric and mood disorders.6
Inflammatory Diseases: Immune function is also influenced by the gut microbiome through interaction with the host’s immuno-response system. Various species of gut bacteria have been shown to promote expression of regulatory T-cells, which are white blood cells that play a central role in the adaptive immune response. Changes in the interaction between the gut microbiome and the host’s immune system have been shown to lead to inflammatory diseases, including inflammatory bowel disease (IBD). Gut bacteria can also indirectly modulate immune function through interaction with invading pathogens.7
Metabolic Diseases: Gut bacteria also play a role in metabolizing consumed food, metabolites, and foreign chemicals as well as harvesting energy by producing vitamins. Metabolites are involved in regulation of glucose levels, and research has shown they are directly linked to metabolic disorders, such as obesity and diabetes.8
One well known therapeutic that focuses on manipulating gut bacteria is FMT for the treatment of recurrent CDI (rCDI), which involves extracting healthy bacteria from a donor’s fecal matter and transferring the bacteria directly into the colon of an infected patient.3 As the microbiome is directly related to the etiology of rCDI, FMT has been accepted by many clinicians, despite its lack of formal FDA approval, as the most effective treatment for patients with rCDI. Occasionally, FMT has been shown to negatively interact with a patient’s existing microbiome, inadvertently introducing disease-causing bacteria.9,10 The range of probiotic therapies advancing through clinical trials seeks to learn from the shortcomings of FMT and provide improved microbiome-based treatment options for rCDI and several other indications.
BARRIERS IN DEVELOPING MICRIOBOME-BASED THERAPEUTICS
Despite the range of promising microbiome-based therapies in development, there remain three key barriers that manufacturers must address to drive these therapeutics towards approval and eventual commercialization. These barriers include the following:
Unsubstantiated Body of Evidence: There is a lack of consensus among industry stakeholders, including physicians, around how altering aspects of the microbiome ultimately impacts human health, and many are calling for additional clinical data demonstrating the safety and efficacy of emerging microbiome-based therapies.10 Some physicians are convinced the microbiome has significant influence on disease pathology and that microbiome manipulation will be critical in addressing a range of diseases, but others believe microbiome-based therapies should not be incorporated in treatment decisions due to the lack of empirical evidence supporting therapeutic use.11 These physicians will have to be convinced of the real-world value microbiome-based therapies provide in order to adopt. Challenges in collecting real-world data also might deter other drug developers from entering the field.
Low Familiarity & Understanding: Currently, most patients and physicians have a limited understanding of these novel products and insight into differentiating factors due to the nascency of the field. Some also do not understand the complexity of the human microbiome or are skeptical of its role in human health. Pending approval, drug developers will need to effectively communicate a product’s value proposition and mechanism of action in a way that resonates with target customers and aligns with their needs.
Undefined Commercial Opportunity: It is unclear exactly how these treatments will be adopted in clinical practice as clinical trials are showing that microbiome-based therapies can be used to address a range of unmet needs. It will be essential to identify the area of highest commercial opportunity in this untapped market where adopters have minimal reference points, but there may be a significant amount of risk for a first-to-market microbiome-based therapeutic.
OPPORTUNITIES TO OVERCOME CRITICAL BARRIERS TO ADOPTION
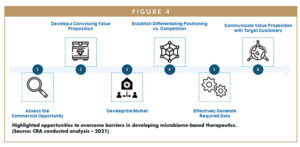
As shown in Figure 4, to successfully drive adoption, developers of microbiome-based therapies will need to overcome the barriers previously outlined and shape current stakeholder opinions by convincing physicians, patients, healthcare providers, payers, and others of the clinical benefits and value of these products. This will require drug developers seeking to enter the field to do so in a systematic manner, following these recommendations:
Assess the Commercial Opportunity: The first step for drug developers is to gain an in-depth understanding of the commercial potential of a product and identify the use that offers the highest commercial potential. This will require the development and application of an opportunity assessment framework, which should encompass defined metrics to assess factors, including (1) the strength of the product’s value proposition; (2) clinical efficacy; (3) the size of the addressable patient population (based on target intervention points in the patient journey); (4) likelihood of physician and patient adoption; (5) level of current and future competitive intensity; and (6) likelihood of payers to cover the product.
Develop a Convincing Value Proposition: Once the opportunity with the highest commercial potential has been identified, developers of microbiome-based therapies must take steps to crystallize the value proposition of their product to ensure it aligns with the unmet needs of target customers, can be sufficiently supported by available clinical data, and resonates with all stakeholders, including physicians, patients, and payers. It will be essential to develop a compelling value proposition in this emerging market to drive product uptake and effectively capitalize on the identified commercial opportunity.
Develop the Market: Another important step is developing the market, which includes building broader awareness and understanding of microbiome-based therapeutic approaches among target stakeholders to ensure potential adopters are willing to try these treatments once available. This may involve providing fundamental background information on what the human microbiome is and how research has shown its role in human health and causing disease. Establishing this baseline familiarity for microbiome-based therapeutics will require implementing a range of market-shaping techniques to convince stakeholders of their clinical value, including education strategy development and awareness campaign execution, to generate demand that supports successful market entry.
Establish Differentiating Positioning Versus Competition: As the microbiome sector gets increasingly crowded by more emerging innovative therapies, coupled with stakeholder skepticism about the clinical benefits they offer, it is essential that developers establish compelling positioning versus the competition. Strategies must differentiate their product from existing standard-of-care treatments and future market entrants. This well help build upon their product’s unique value proposition to support uptake post-launch.
Effectively Generate Required Data: Lack of supporting clinical data is a primary challenge in the adoption of microbiome-based therapies, highlighting the need to build a comprehensive evidence generation strategy. Developers will need a guide for how to develop the level of clinical data required by regulators and preferred by customers, potentially going beyond data from ongoing clinical trials. An evidence generation plan should detail how to identify data gaps relevant to a product’s value proposition (eg, patient outcomes, health economic and outcomes research data), prioritize these data gaps and generate the data needed to demonstrate a product’s clinical benefits, value proposition, and positioning. Executing an evidence generation strategy will likely require developers to pursue a range of activities, such as conducting retrospective claims analyses, prospective chart studies, and additional clinical trials as efficiently as possible.
Communicate Value Proposition With Target Customers: When all required data are generated and collected, developers of microbiome-based therapies must then strategically communicate a product’s value proposition with target customers using marketing and field efforts. This might include providing real-world payer or physician testimonials to customers, positioning sales teams to address physician concerns about a product’s value and clinical benefits and developing patient FAQs and payer budget impact models. All activities should be tailored to each product and the needs of each customer to ensure they are targeted and cost-effective, and that the value proposition resonates with key stakeholders.
Coordination of successful development and commercialization for emerging microbiome-based therapies requires strategic execution of these tactics. Developers might consider partnering with experts who have experience in clinical development of innovative technologies and understand what it takes to successfully drive product uptake with customers post-launch. Developers in the microbiome sector should also observe their competitors’ strategies, assess the benefits and risks of each and apply these learnings to their own operations.
SUMMARY
When identifying promising microbiome therapeutic approaches for any disease or condition, it is critical to understand the mechanism of disease and disease pathways as well as the role of the microbiome. While there has been increasing evidence that a healthy microbiome supports proper function of organs and metabolic systems, more research needs to be done to further establish the connection between the microbiome and human health. Advances in research will help drive a new focus on the treatment of diseases linked to the microbiome and the emergence of novel microbiome-based therapies, including probiotics, in the years ahead. Manufacturers must carefully consider any existing barriers and the optimal approach for entering the microbiome field to ensure success.
The views expressed herein are the authors’ and not those of Charles River Associates (CRA) or any of the organizations with which the authors are affiliated.
REFERENCES
- de Vos, W. M., & de Vos, E. A. (2012, August 1). Role of the intestinal microbiome in health and disease: from correlation to causation. Nutrition Reviews, 70(1), S45-S56.
- McCune et al (2020). Results from the first English stool bank using faecal microbiota transplant as a medicinal product for the treatment of Clostridioides difficile infection. EClinical Medicine. DOI: https://doi.org/10.1016/j.eclinm.2020.100301.
- Gupta, S., Allen-Vercoe, E., & Petrof, E. (2016, Mar). Fecal microbiota transplantation: in perspective. Gastroenterology, 9(2), 229-39.
- Group, M. T. (2020). Microbiome Therapeutics Innovation Group Statement Safety Alert Regarding Use of Fecal Microbiota for Transplantation and Risk of Serious Adverse Events Likely Due to Transmission of Pathogenic Organisms. Retrieved from https://microbiometig.org/statements/.
- Marks, L. (2018, March). The human microbiome. What Is Biotechnology. Retrieved from https://www.whatisbiotechnology.org/index.php/science/summary/microbiome/the-human-microbiome-refers-to-the-complete-set-of-genes.
- Ghaisas, S., Maher, J., & Kanthasamy, A. (2016, February). Gut microbiome in health and disease: Linking the microbiome–gut–brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacology & Therapeutics, 158, 52-62.
- Vanderhoof, J. (2017, March). 147 Early changes in the human Microbiome alter immune function and immunologically mediated disorders. Journal of Animal Science, 95(2), 69-70.
- Wischmeyer, P., McDonald, D., & Knight, R. (2016). Role of the microbiome, probiotics, and ‘dysbiosistherapy’ in critical illness. Current Opinion in Critical Care, 22(4), 347-353.
- Wong, A., & Levy, M. (2019, June). New Approaches to Microbiome-Based Therapies. mSystems.
- FDA. (2016, March). Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. Retrieved from https://www.fda.gov/ regulatory-information/search-fda-guidance-documents/enforcement-policy-regarding-investigational-new-drug-requirements-use-fecal-microbiota-0.
- Adolph, T., Grander, C., Moschen, A., & Tilg, H. (2018, May). Liver-microbiome axis in health and disease. Trends Immunology, 39(9), 712-723.
- Tilg, H., & Moschen, A. (2015, May). Food, immunity, and the microbiome. Gastroenterology, 148(6), 1107-1119.

Andrew Thomson is a consulting associate within the Life Sciences Practice at CRA with 3+ years of experience in commercial strategy consulting with pharmaceutical and biotech clients alike, focused on microbiome-based healthcare solutions.

Brian Carpenter is a principal in the Life Sciences Practice at CRA with 10+ years of biotech experience, including extensive experience in commercialization strategy for microbiome-based therapeutics and diagnostics.

Robert Broadnax is a vice president in the Life Sciences Practice at CRA with 20+ years of commercialization experience, focused on commercial strategy for microbiome-based therapeutics and diagnostics, as well as prior brand leadership and executive experience at Bristol-Myers Squibb, Roche, UCB & TRM Oncology.
Total Page Views: 5488