Issue:April 2024
DRUG DELIVERY PLATFORM - VitalDose® EVA Implants for Systemic & Local Delivery of Therapeutics
LIMITATIONS OF CONVENTIONAL DELIVERY METHODS
As the pharmaceutical development landscape continues to evolve, the search for effective drug delivery technologies continues to be a major driver of innovation. Traditional drug delivery methods, such as oral and injectable formulations, have long been the foundation of therapeutic treatment. However, these approaches have limitations when delivering peptides, biologics, and RNA therapeutics. Innovative drug delivery technologies can help improve drug efficacy, address toxicity issues, and improve patient compliance, which all have potential to improve treatment outcomes.
Systemic delivery approaches that leverage continuous dosing can address adherence issues and improve drug effectiveness while minimizing adverse reactions. Additionally, a localized delivery approach can minimize total drug exposure, reduce off-target toxicities, and overcome targeting issues.
VITALDOSE® EVA IMPLANTS FOR DELIVERY
Polymer-based, durable implants have the potential to overcome the challenges associated with traditional delivery methods for both systemic and localized applications. By providing sustained, continuous dosing, implants may result in better therapeutic outcomes over therapies which require more frequent administration. Because biodurable solutions are not inert and do not degrade, they do not create any degradative byproducts that may cause safety issues.
Durable implants like those composed of VitalDose® EVA, offer tunable parameters including the following:
- High loading (up to 70%) to incorporate large doses and achieve desired drug release per day
- Compatibility across a wide range of molecules from small molecules and peptides to monoclonal antibodies (mAbs) and RNAi therapeutics
- Customized release profiles from months to years
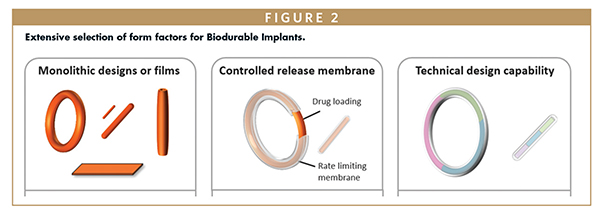
- Extensive selection of form factors and geometries; from rods and rings to films and complex configurations
Biodurable implant solutions can be refilled, replaced, or retrieved (in the case that therapy needs to be interrupted) to achieve systemic or localized delivery across a wide range of therapeutic areas.
SYSTEMIC DELIVERY
Two attributes of EVA which have made it well suited for delivering drug molecules systemically are (1) its high drug loading capability and (2) its straightforward mechanisms for fine tuning drug-release rates. Examples of such mechanisms include changing the vinyl acetate-to-ethylene ratio of the polymer, as well as modifications to the polymer core and membrane formulation design of the implant. These attributes provide continuous systemic dosing over months to multiple years while avoiding an API burst soon after administration, which can be undesirable for some disease treatments.
WOMEN’S HEALTH
EVA has been used for decades in two contraceptive therapeutics for women. The commercial revenue of these products demonstrates a strong desire from patients for convenient therapeutics with a low dosing frequency.
Nexplanon®, a 2-mm diameter x 4-cm length rod, is implanted via a trocar into the subcutaneous tissue of the upper arm. The therapeutic efficacy of Nexplanon has been observed to be more than 99% effective and it is forecasted to achieve over $1 billion in sales in 2025.1,2 The product is currently labeled for 3 years of use, but clinical studies are ongoing to study efficacy at 5 years.3 NuvaRing® is the second contraceptive therapeutic composed of an EVA ring. This ring is self-administered intravaginally every 4 weeks. One benefit of NuvaRing versus oral contraceptive pills is its superior cycle control, which is attributed to its more consistent daily serum levels from continuous dosing, as opposed to daily dosing (NuvaRing daily dosing level of ethyinyl estradiol is half that of oral pill: 15 mcg versus 30 mcg).4 NuvaRing reached peak sales of $902 million.5,6
These commercially well-established contraceptive products have led to EVA’s inclusion on the US FDA inactive ingredient database (IID), creating a platform of multiple dosage forms that can be leveraged to deliver drug molecules systemically for therapies other than contraception.7 For example, an intravaginal ring dosage form is currently being clinically evaluated as a Multi-preventative Purpose Technology (MPT) to provide both contraception and protection against HIV.8 Intravaginal rings for endometriosis treatment and menopause symptoms are also under investigation.9,10
ONCOLOGY
Additional opportunities to improve therapeutic delivery also exist in the realm of delivering systemic adjuvant therapy in oncology. As an example, sustained delivery of hormone therapy can increase patient adherence for both breast and prostate cancer patients. Improved adherence of this type of therapy for both indications can lead to decreased risk of cancer recurrence as well as lower rates of tumor progression. In addition, a lower recurrence risk is linked to increased overall survival rates compared to patients that are less adherent to their therapy. Ultimately, this leads to lower healthcare costs as there is a reduced need for additional treatment interventions and hospitalizations that would be a result of treatment failure.11
CENTRAL NERVOUS SYSTEM DISORDERS
Central nervous system (CNS) disorders are often characterized by hard-to-reach targets where drug transport across the blood brain barrier is frequently needed to realize true therapeutic efficacy. In a disease like multiple sclerosis (MS), a range of therapeutic strategies are employed depending on MS type and severity. Treatments range from daily oral medications to longer-acting infusions and injections. For Relapsing-Remitting MS (RRMS) patients with moderate disease taking daily orals, there are limited long-acting options; subcutaneous implants can provide a convenient solution to reduce treatment burden. Reformulations of small molecule oral drugs, like fingolimod or ozanimod, into implants inserted every 6 months, would improve patient convenience and minimize the reminder of disease burden for the patient. For RRMS patients with severe disease, the high dosing requirements and treatment burden associated with some monoclonal antibody infusions could be mitigated by a continuous, lower-dosed implant solution. This approach is not limited to MS. Therapies for other conditions, like Alzheimer’s, are often delivered at frequent, high doses to overcome physiological, blood-brain barrier issues. Alzheimer’s treatments (approved products and those in development) that are dosed twice a month could be improved with a patient-centric subcutaneous implant with continuous dosing.
LOCALIZED DELIVERY
Localized drug delivery via an implant has the potential to overcome challenges associated with delivering drugs to difficult target sites. Chronic conditions afflicting sites like the eye, brain, or solid tumors face challenges with frequent injections or infusions, large doses, and physiological barriers preventing continuous exposure to the drug at the target site. By situating the implant at or near the target site, a continuous dose can be delivered to maintain therapeutic effect for a prolonged period. This approach may also allow for lower dosing as compared to repeated administration of bolus drug loads.
OPHTHALMOLOGY
In chronic ocular conditions, an implant that elutes drug for over six months mitigates the treatment burden associated with daily eye drops or frequent intravitreal injections. Durable implants, like Iluvien™ for retinal conditions, offer drug delivery for up to 3 years and similar approaches are in development for the treatment of wet age-related macular degeneration.12,13 Continuous delivery from an implant may also provide increased drug exposure when delivering to areas like the suprachoroidal space. EVA has been used in commercialized ophthalmic implants (e.g., Ocusert, Vitrasert®, iDose® TR) and is currently under investigation for suprachoroidal therapeutics.14
Delivery of mAbs, small molecules, or RNAi therapeutics to relevant compartments in the eye offers a patient-centric solution to address low adherence.15 The proximity of the implant to the target site potentially improves bioavailability and reduces side effects by avoiding drug washout and off-target delivery. Durable implants mitigate tear turnover, blinking, and corneal and conjunctival barrier issues that result in low therapeutic efficacy of eye drops. Recently approved iDose TR (Glaukos), is a ~0.5-mm diameter by 1.8-mm implant that incorporates a VitalDose EVA membrane into a titanium implant structure.16 The VitalDose EVA membrane allows for continuous prostaglandin release and is designed to deliver up to 3 years of drug therapy.17 Biodurable treatment approaches may also lessen healthcare resource utilization and cost burden associated with frequent visits.18
ONCOLOGY
In a similar fashion, localized drug delivery in oncology has been gaining strong momentum in the treatment of solid tumors across multiple indications. The localization via an implant dosage form can provide physical targeting of the drug directly to the tumor site (Figure 1). Not only can this enhance the delivery of oncology therapeutics that lack molecular targeting abilities, but this can also compliment drugs with built-in targeting, such as antibody or peptide drug conjugates or bispecific antibodies, enhancing their molecular targeting with the added physical targeting benefit. This will allow the confinement of the treatment to the site of the disease which can ultimately minimize total drug needed while maximizing efficacy and reducing adverse effects.19
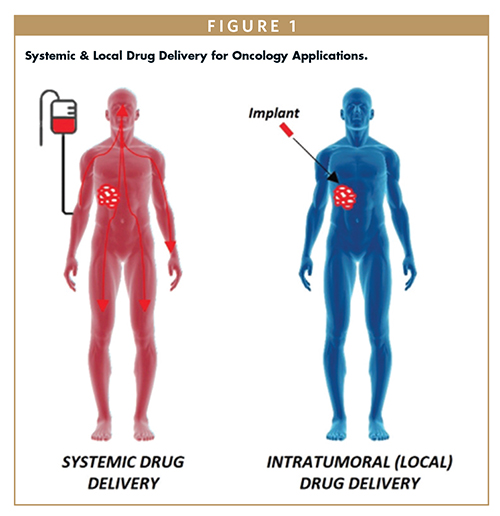
In addition, an implant delivery system can provide modified release kinetics to slowly release drug into the tumor to circumvent rapid tumor leakage that is often seen with repeated intratumoral injections.20
SUMMARY
Traditional drug delivery methods (oral and injections) face limitations within today’s landscape of complex drug devel opment. These limitations include but are not limited to stability, absorption, and degradation issues related to oral administration and frequency of injection administration. VitalDose EVA implants offer a valuable solution by providing sustained, continuous dosing that can overcome formulation limitations. Its capabilities for high drug loading enables medication to last for months or even years which can significantly reduce the burden of frequent dosing while optimizing patient freedom and adherence. In addition, VitalDose EVA demonstrates broad compatibility with a wide range of drug molecules and possesses significant design flexibility to suit different administration routes for patient-centric drug products.
VitalDose implants have been commercially validated in both contraceptive and ophthalmic indications, and the drug delivery platform shows further promise across a wide range of indications for both systemic and localized drug delivery.
Nexplanon® and NuvaRing are registered trademark of N.V. Organon; Celanese is not affiliated with nor sponsored by N.V. Organon.
REFERENCES
- “Overview.” Organon, 2024, https://organonpro.com/en-us/product/nexplanon/overview/ Accessed 26 January 2024.
- “Nexplanon: Product Overview Summary”. Evaluate Pharma. Accessed 26 January 2024.
- A Study to Assess Contraceptive Efficacy and Safety of Etonogestrel (ENG) Implant Beyond 3 Years of Use (MK-8415-060), NCT04626596. Clinicaltrials.gov, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04626596 Accessed 26 January 2024.
- Roumen FJ. Review of the combined contraceptive vaginal ring, NuvaRing. Ther Clin Risk Manag. 2008 Apr;4(2):441-51. doi: 10.2147/tcrm.s1964. PMID: 18728840; PMCID: PMC2504064.
- Organon & Co. 2021. 99.1 Exhibit Statement. https://www.sec.gov/Archives/edgar/data/1821825/000119312521084081/d56612dex991.htm.
- Evaluate Pharma. NuvaRing Reported Annual Historic Sales. Accessed February 2024.
- Inactive Ingredients Database Download. U.S. Food & Drug Administration website. https://www.fda.gov/drugs/drug-approvals-and-databases/inactive-ingredients-database-download. Accessed February 2, 2024.
- Population Council. (2023 September 12). Celanese VitalDose® Drug Delivery Platform Will Enable the Population Council’s Contraceptive and HIV Multipurpose Prevention Technology [Press release]. https://popcouncil.org/media/celanese-announces-agreement-with-the-population-council-for-sustained-release-dual-api-therapeutic/.
- Vercellini P, Barbara G, Somigliana E, Bianchi S, Abbiati A, Fedele L. Comparison of contraceptive ring and patch for the treatment of symptomatic endometriosis. Fertil Steril. 2010 May 1;93(7):2150-61. doi: 10.1016/j.fertnstert.2009.01.071. Epub 2009 Mar 27. PMID: 19328469.
- “Our Pipeline: DARE-HRT1”. Dare Bioscience, 2024, https://darebioscience.com/pipeline/dare-hrt1/ Accessed 26 January 2024.
- National Cancer Policy Forum; Board on Health Care Services; Institute of Medicine. Delivering Affordable Cancer Care in the 21st Century: Workshop Summary. Washington (DC): National Academies Press (US); 2013 May 20. POSSIBLE SOLUTIONS. Available from: https://www.ncbi.nlm.nih.gov/books/NBK202467/.
- Frances E Kane , Judith Burdan , Antonio Cutino & Kenneth E Green (2008) Iluvien™: a new sustained delivery technology for posterior eye disease, Expert Opinion on Drug Delivery, 5:9, 1039-1046.
- Seah, I et al. Use of Biomaterials for Sustained Delivery of anti-VEGF to treat retinal disease. Eye (2020) 34:1341-1356.
- Celanese. (2023 February 14). Celanese Announces a Research Agreement with Johns Hopkins University to Advance Sustained Ocular Drug Delivery to the Suprachoroidal Space [Press release].
- Tapply I, Broadway DC, “Improving Patient Adherence to Topical Medication in patients with Glaucoma”. Patient Prefer Adherence, 2021, 15, pp 1477–1489.
- Celanese. (2023 April 4). Celanese Announces Agreement with Glaukos Corporation for Sustained Release Glaucoma Treatment [Press release].
- Berdahl, John P et al. “Efficacy and Safety of the Travoprost Intraocular Implant in Reducing Topical IOP-Lowering Medication Burden in Patients with Open-Angle Glaucoma or Ocular Hypertension.” Drugs vol. 84,1 (2024): 83-97. doi:10.1007/s40265-023-01973-7.
- Shih, V et al. Clinical and Economic Burden of Glaucoma by Disease Severity. American Academy of Ophthalmology. 2021.
- Ying Xuan Chua C, Ho J, Demaria S, Ferrari M, Grattoni A, “Emerging Technologies for Local Cancer Treatment”. Advanced Therapeutics, 2020 Sep, 3 (9): 2000027. https://doi.org/10.1002/adtp.202000027.
- Liu H, Gonzalez DD, Viswanath DI, Vander RS, et al., “Sustained Intratumoral Administration of Agonist CD40 Antibody Overcomes Immunosuppressive Tumor Microenvironment in Pancreatic Cancer”. Advanced Science, 2023, 10, 202206873. https://doi.org/10.1002/advs.202206873.

Dr. Cyonna Holmes earned her PhD in Biomedical Engineering from the University of Texas Southwestern Medical Center (Dallas, TX) and her BS in Bioengineering from Stanford University (Stanford, CA). At Celanese, she serves as Global Strategy Lead for Ophthalmology, Rare Diseases, and RNA Therapeutics. She has experience in the areas of drug delivery, lifecycle management strategy, new product development, and market entry strategy. She has published articles in peer-reviewed journals.

Karen Chen earned her MS in Formulation Science from Fairleigh Dickinson University (Teaneck, NJ) and her BS in Biology and Chemistry from Rutgers University (New Brunswick, NJ). At Celanese, she serves as Global Strategy Lead for Oncology, Obesity, mAbs and peptides. Her experience spans new product development as well as market entry and expansion strategies for functional excipients and drug delivery.

Brian Duke earned his BS in Industrial Engineering from Texas A&M University (College Station, TX) and his MBA from Pennsylvania State University (State College, PA). At Celanese, he serves as Global Strategy Leader for Women’s Health and Bioresorbable Technologies. His professional experiences have primarily focused on marketing & sales of polymers for pharmaceutical and industrial applications, as well as business strategy development focusing on external partnerships and operating models.
Total Page Views: 4649











