Issue:May 2019
DRUG DELIVERY – ENHANZE (®): An Efficient Way to Optimize Biologic Therapies for Subcutaneous Administration
ABSTRACT
Halozyme Therapeutics, Inc. has developed the ENHANZE® drug delivery technology that enables and optimizes subcutaneous drug delivery for a range of therapies typically administered intravenously or by frequent subcutaneous injection. The technology uses Halozyme’s patented enzyme recombinant human hyaluronidase PH20 (rHuPH20) that acts locally and transiently to degrade hyaluronan (HA) in the extracellular matrix. Degradation of HA in the subcutaneous space with rHuPH20 allows for a larger injection volume than is possible with traditional subcutaneous delivery. This results in increased dispersion and absorption of subcutaneously co-administered injected drugs and fluids, thus enabling the delivery of targeted therapies for oncology, autoimmune conditions, hematologic diseases, and other disease states. The transition from intravenously administered therapies to subcutaneous delivery and the optimization of subcutaneous delivery have been demonstrated to improve patient experiences, increase efficiency in drug administration, and reduce overall healthcare costs.
INTRODUCTION
Biologics are revolutionizing modern medicine by providing new approaches for treating diseases, such as cancer, autoimmune diseases, and other chronic or inheritable conditions. Biotherapeutics are also the fastest-growing sector in the pharmaceutical industry; in 2016, 8 out of the top 10 best-selling drugs worldwide were biologic agents.1 Recent market research has indicated that the global market for biologic drugs may reach $580.5 billion in sales by 2026.2
Although biologic therapies offer the potential for tremendous progress in medical care, challenges may exist in terms of administration routes, formulation properties, dosing regimens, and safety. Large molecule biologics often require administration of large doses, which results in many of these biologic therapies being delivered by intravenous infusion, with only some designed for multiple and/or subcutaneous self-injections. The need for parenteral administration of these agents may also limit the development of new biologic therapies because of restrictions on viscosity, pH, and osmolality.3
Halozyme Therapeutics, Inc., a biotechnology company based in San Diego, CA, has developed a novel drug delivery technology (ENHANZE drug delivery technology) that may overcome some of the challenges associated with subcutaneous drug delivery. Subcutaneous administration of therapeutics may enable a better experience for patients and has the potential to increase healthcare system efficiencies and decrease overall healthcare expenses.4
HOW RHUPH20 WORKS
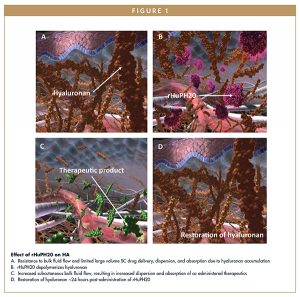
The subcutaneous space comprises the extracellular matrix and various cell types, such as adipocytes, fibroblasts, and macrophages. The fibrous network contains both long-lasting structural components, such as collagen and elastin, and short-lived components, such as hyaluronan (HA).5 Hyaluronan, a naturally occurring glycosaminoglycan with repeating disaccharide subunits of D-glucuronic acid and N-acetyl-D glucosamine, is a crucial component of the extracellular matrix. Hyaluronan controls tissue water content and is crucial for cellular homeostasis.6 Approximately 30% of HA (up to 15 g distributed across the body) is replaced on a daily basis.6 Hyaluronan acts as a barrier to prevent bulk fluid flow and is one of the reasons why subcutaneous drug delivery methods are typically restricted to small-volume injections.7
Central to the ENHANZE drug delivery technology is Halozyme’s proprietary recombinant human hyaluronidase PH20 enzyme, rHuPH20, which works locally and transiently to degrade HA in the extracellular matrix of the subcutaneous space by cleaving the linkage between the disaccharide units that comprise HA.7 Hyaluronan is restored at the local site within 18 to 24 hours after injection with rHuPH20.8,9 By temporarily degrading HA at the injection site, rHuPH20 enables subcutaneous bulk fluid flow and increased dispersion and absorption of co-administered therapeutics. The ENHANZE drug delivery technology may enable reformulation of existing intravenous (IV) drugs for subcutaneous injection. Additionally, rHuPH20 may be added to existing subcutaneous drug formulations to optimize dosing and administration, providing the opportunity for a wide range of therapies to have expanded delivery options beyond IV infusions or multiple subcutaneous injections.
REDUCING THE TREATMENT BURDEN FOR PATIENTS
Owing to the increased costs of administering drugs intravenously versus subcutaneously or intramuscularly, it may be advantageous to further develop subcutaneous drug delivery approaches for biological agents.10 The ENHANZE drug delivery technology can potentially reduce the time and/or the frequency of dosing required for IV administration or subcutaneous injection as compared with those same treatments when they do not use rHuPH20.
STUDIES EVALUATING THE DELIVERY OF THERAPEUTIC AGENTS IN COMBINATION WITH RHUPH20
Although the ENHANZE drug delivery technology can aid in the subcutaneous administration of drugs for a broad range of therapeutic areas, several current applications involve co formulations with oncology therapies. Several studies have found that rituximab (MabThera®) and trastuzumab (Herceptin®) co-formulated with rHuPH20 for subcutaneous administration demonstrated reduced administration times compared with IV formulations while maintaining similar safety and efficacy profiles.11 Recent data presented at ASCO 2018 from an investigational study in patients with multiple myeloma demonstrated that a subcutaneous formulation of daratumumab with rHuPH20 enabled a dosing time of 3 to 5 minutes compared with a typical IV administration time of 4 to 6 hours.12
One study compared the time commitment required for IV delivery of the biologic drug trastuzumab versus the time commitment for a subcutaneous injection of trastuzumab with rHuPH20. Trastuzumab is a targeted therapy that is used to treat HER2-positive breast cancer in both early and metastatic settings.13 Patients treated with intravenous trastuzumab typically receive infusions lasting 30 to 90 minutes. By contrast, the subcutaneous formulation of trastuzumab with rHuPH20 can be administered within 2 to 5 minutes.4,14
Another study evaluated the treatment preferences of patients with HER2-positive breast cancer who received trastuzumab either through the standard IV formulation (n=235) or the subcutaneous formulation with rHuPH20 (n=232). Results indicated that 88.9% of patients preferred the subcutaneous formulation, 9.6% preferred the IV formulation, and 1.5% had no preference. “Time saving” was given as the main reason for preferring the subcutaneous formulation over the IV formulation.15
Patients with primary immunodeficiency disease (PID) generally prefer subcutaneous injections to IV administered drugs; however, subcutaneous injections of immunoglobulin (Ig) replacement therapy require weekly or biweekly small-volume infusions (15 to 30 mL) at multiple injection sites.16 rHuPH20 can be used to improve the dispersion of Ig replacement therapy, thereby enabling the subcutaneous administration of large volumes (up to 300 mL) of Ig at a single site and potentially enabling the interval between treatments to be expanded from weekly/biweekly to every 3 to 4 weeks.17,18
Early collaborations with Roche and Baxter (now Takeda) have been followed by more recent clinical research efforts in partnership with Pfizer, Baxter (now Takeda), Janssen, AbbVie, Eli Lilly, Bristol-Myers Squibb, and Alexion using the ENHANZE drug delivery technology.19,20
SUMMARY
Drug developers are working to develop new products and improve existing therapies to help patients manage their diseases effectively while reducing their treatment burden.
The ENHANZE drug delivery technology has the potential to improve the pharmacokinetic profiles of co-administered drugs through increased dispersion, absorption, and bioavailability. Also, it may have an important role in reducing treatment burden by enabling at-home self-administration or by decreasing administration times and allowing patients to undergo treatment in a physician’s office instead of at an infusion center.16,18 Furthermore, the use of the ENHANZE drug delivery technology may help reduce local swelling and induration when compared with subcutaneously administered therapeutics that do not contain rHuPH20.17 All these benefits could lead to increased health system efficiencies by reducing administration time versus IV drug delivery, resulting in increased patient throughput, reduced product waste, and decreased overall healthcare expenses.
Although the subcutaneous delivery of therapeutic agents has long been explored as a means to deliver biologic agents more effectively and efficiently, the presence of HA in the subcutaneous space has thus far prevented large volumes of liquid from being injected and quickly absorbed. The patented enzyme rHuPH20, which is the central component of the ENHANZE drug delivery technology, has proven effective in addressing this challenge by temporarily ENHANZE drug delivery technology may enable large volumes of biologics and other agents to be rapidly delivered to their relevant target tissues and has the potential to improve the drug delivery experience for a wide range of patients.
REFERENCES
- Ngo HX, Garneau-Tsodikova S. What are the drugs of the future? Medchemcomm. 2018;9:757-8.
- Biologics Market is Expected to Grow at the CAGR of 9.5% During 2018-2026. 2018. (Accessed July 20, 2018, at https://globenewswire.com/news-release/ 2018/04/16/1471980/0/en/Biologics-Market-is-Expected-to-Grow-atthe-CAGR-of-9-5-During-2018-2026-Credence-Research.html).
- Škalko-Basnet N. Biologics: the role of delivery systems in improved therapy. Biologics. 2014;8:107-14.
- Jin JF, Zhu LL, Chen M, et al. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence. 2015;9:923-42.
- Turner MR, Balu-Iyer SV. Challenges and Opportunities for the Subcutaneous Delivery of Therapeutic Proteins. J Pharm Sci. 2018;107:1247-60.
- Viola M, Vigetti D, Karousou E, et al. Biology and biotechnology of hyaluronan. Glycoconj J. 2015;32:93-103.
- Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4:427-40.
- Hompesch M, Muchmore D, Morrow L, Vaughn D. Accelerated Absorption and Reduced Glycemic Variability in T1DM Patients Human Hyaluronidase-Facilitated Coinfusionof a PrandialInsulin Analog. European Association for the Study of Diabetes; 2011; Lisbon, Portugal. p. 1043.
- Morrow L, Muchmore D, Hompesch M, Jiang P, Jadin L, Vaughn D. Addition of Human Hyaluronidase to Rapid Analog Insulin Reduces the Absolute Variability of Early Insulin Absorption across Infusion Set LifeLINDA. American Diabetes Association Scientific Sessions. San Diego, CA2011:Abstract 27-LB.
- Tetteh EK, Morris S. Evaluating the administration costs of biologic drugs: development of a cost algorithm. Health Econ Rev. 2014;4:26.
- Shpilberg O, Jackisch C. Subcutaneous administration of rituximab (MabThera) and trastuzumab (Herceptin) using hyaluronidase. Br J Cancer. 2013;109:1556-61.
- Ajai Chari, Saad Zafar Usmani, Maria-Victoria Mateos, et al. Subcutaneous daratumumab (DARA) in patients (Pts) with relapsed or refractory multiple myeloma (RRMM): Part 2 update of the open-label, multicenter, dose escalation phase 1b study (PAVO). J Clin Oncol 2018;36:suppl; abstr 8013.
- HERCEPTIN Prescribing Information. Genentech, Inc., South San Francisco, CA. 2017. (Accessed September 25, 2018, at https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf).
- Sanford M. Subcutaneous trastuzumab: a review of its use in HER2-positive breast cancer. Target Oncol. 2014;9:85-94.
- Pivot X, Gligorov J, Muller V, et al. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two cohort PrefHer study. Ann Oncol. 2014;25:1979-87.
- Wasserman RL. Overview of recombinant human hyaluronidase-facilitated subcutaneous infusion of IgG in primary immunodeficiencies. Immunotherapy. 2014;6:553-67.
- Kang DW, Jadin L, Nekoroski T, Drake FH, Zepeda ML. Recombinant human hyaluronidase PH20 (rHuPH20) facilitates subcutaneous infusions of large volumes of immunoglobulin in a swine model. Drug Deliv Transl Res. 2012;2:254-64.
- Wasserman RL, Melamed I, Stein MR, et al. Recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulins for primary immunodeficiency. J Allergy Clin Immunol. 2012;130:951-7 e11.
- ENHANZE® Drug Delivery Technology. 2018. (Accessed November, 2018, at https://www.halozyme.com/technology/enhanze/default.aspx).
- Baxter Launches HYQVIA in the United States for Adult Patients with Primary Immunodeficiency. 2014. (Accessed November, 2018, at https://www.halozyme.com/investors/news-releases/news-release-details/2014/Baxter-Launches-HYQVIA-in-the-United-States-for-Adult-Patientswith-Primary-Immunodeficiency/default.aspx).
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Michael J. LaBarre is Vice President of Product Development at Halozyme. His career comprises more than 2 decades of biotechnology drug discovery and development experience, including work on successful approvals of commercial therapies such as Rituxan® in 1997 and more recently, RITUXAN HYCELA® with Roche in 2017. Prior to joining Halozyme in 2008, he served as Vice President, Product Development at Paramount BioSciences LLC; Director, Analytical and Protein Biochemistry, Discovery Research at Biogen Idec; and Director, Analytical and Formulation Sciences, Research and Development at IDEC Pharmaceuticals. He earned his BS in Chemistry from Southampton College of Long Island University and his PhD in Bioinorganic Chemistry from the University of Arizona.
Total Page Views: 8191