Issue:November/December 2019
CONTINUOUS MANUFACTURING - Continuous Manufacturing in Pharmaceuticals: Implications for the Generics Market
Continuous manufacturing (CM) is a new trend in manufacturing. From paper to petrochemicals and automobiles, industries are embracing continuous manufacturing to enhance efficiencies and increase profits. The highly regulated nature of the pharmaceutical sector, and the low-risk-taking nature of this market, are the two main factors that have restrained the implementation of novel methods of manufacturing by pharmaceutical industries. However, the burgeoning demand for complex and innovative therapies and rising competition have led pharmaceutical manufacturers to reconsider their methods of manufacturing.
The age-old batch manufacturing process of making drugs is still the most popular method for both branded and generic drug manufacturers. The drugs are produced in single large batches step by step. A typical pharmaceutical batch manufacturing facility is built with an investment of billions of dollars and contains multiple pieces of complicated equipment. The employment of human labor and multiple transfer steps poses extreme contamination risks and introduces the possibility of errors.
Considering the commercial advantages offered by CM, the pharmaceutical industry is becoming increasingly receptive to this technology. CM technology offers a number of benefits to the pharmaceutical industry:
-A CM processing facility is at least 70% smaller than a batch production facility. The reduced size of equipment and the overall facility greatly saves operational, running, and other environmental costs.
-CM processes are highly advantageous for the production of compounds with harmful intermediates and those that may be prone to degradation.
-The growing popularity of personalized medicine and the reduction in batch sizes are proving to be burdensome for pharmaceutical manufacturers. Smaller batch production does not justify the expensive equipment and associated infrastructure costs. In contrast, it is much more economical to use smaller, single-use equipment in a continuous mode that would quicken the supply chain and boost productivity.
-From a commercial and regulatory standpoint, CM is beneficial to pharma manufacturers because the same equipment that has been used during process development can be employed for production scale. Scaling up is much easier in a continuous set-up, which eliminates any validation issues and saving costs.
-CM is also an effective solution for multi-step reactions that are commonplace in the pharmaceutical industry.
-Another advantage that CM technology offers is its amenability to automation. The integration of real-time sensors and measuring equipment with continuous manufacturing equipment enables continued monitoring and feedback control for the processes. The continued generation of process data enables manufacturers to analyze the data and use them to bring improvements to the processes. In addition to providing better quality products and improved productivity, real-time process control supports the FDA’s quality-by-design (QbD) approach of manufacturing.
Throughout the years, product recalls due to poor drug production practices or inferior drug quality have increased exponentially; this comes as demand for drugs is rising sharply. Concerns lurk among pharmaceutical suppliers when there is an upsurge in demand for a particular drug. In both these situations, CM is the go-to strategy. Using CM technology, any response to market changes can be enacted quickly, eliminating many issues related to drug shortages.
In July 2015, the first drug produced by a CM process was Orkambi by Vertex Pharmaceuticals. Orkambi is a cystic fibrosis oral solid dosage (OSD) drug produced using a continuous manufacturing process. Since then, many pharmaceutical companies have forayed into the continuous manufacturing domain. FDA’s support and the go-getter attitude of pharmaceutical companies have led to a total of four approvals for drugs produced using CM. At least 20 companies are talking to the FDA’s staff about developing and implementing CM processes. The table below lists the drugs that have been produced by continuous manufacturing and received FDA approval.
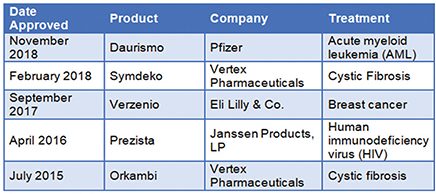
FDA-Approved Continuously Manufactured Drugs. Source: BCC Research
The changing attitude of branded drug companies to using CM in their production processes is evident from the five drugs approved by the FDA and the fact that many more companies are actively engaged in discussions with the FDA and other suppliers to allow the implementation of CM in their processes. In contrast, generic drug manufacturers are treading the path very carefully because of the difference in the way the generics market works — it is based on a much different business model. Regulatory policies, competitive environments, manufacturing strategies, and barriers to entry and exit work in different ways for the generics sector.
In the case of branded drugs, manufacturers have extended exclusivity periods reaching up to 15 years with extensions, thereby giving them the opportunity to recover the costs of research and development with greater ease. However, for generic drug manufacturers, the market is already competitive with the brand’s popularity and usage habit with its consumers. The first generic entrant though gets a limited period of exclusivity, generally 180 days, but that is soon lost, making way for more competitors to enter the market. In this scenario, the demand for a particular generic drug is always unpredictable and highly variable. In the case of uncertainty in future demand and no guarantee of return on investment, it is of no interest to generic drug manufacturers to invest heavily in new manufacturing technologies.
CM has been shown to possess a large cost-reducing potential. A study by Schaber, et al, estimated that capital expenditures would be between 20% and 76% lower in a CM facility and that overall costs would be 9% to 40% lower in a CM facility manufacturing a single blockbuster drug using dedicated equipment.1 Pfizer transitioned its Lipitor production process to a “hybrid” approach based on CM technology; however, the company’s experience was not satisfactory. Pfizer’s CEO later said that to realize the full benefits from investments in CM, high-volume production is needed.
In the case of generics, if manufacturers face unpredictability in demand, and return on investment is also uncertain, it is highly unlikely that they will invest in the cost-intensive transformation of the existing batch facilities to continuous production facilities. In addition to new equipment and facility design, CM warrants the investment in novel PAT tools and technologies that will allow monitoring and control of the processes in real-time. Labor requirements are also intensified as highly skilled and trained personnel are required. Moreover, the risks and costs associated with new validations and regulatory approvals also add to the overall investment.
Another cost-related issue particularly relevant for generics is the value associated with their existing facilities and equipment. According to data released by Standard and Poor’s Capital IQ Financial on the top 20 generic pharmaceutical companies, the average lifespan of depreciable assets (machinery and buildings) of these companies ranges from 4 to 12 years. As such, the abandonment of functional equipment does not make sense until the profit margins are high enough. Only when CM technology can prove its robustness and sustainability worthy of investment will it attract generic drug manufacturers.
Generics companies operate within low margins, so cost of manufacturing is an important factor when considering the competition; this is one of the main reasons why generic drug manufacturers are not changing over to CM. The costs and time associated with transformation from a well-established batch process to a new and still developing CM process may pose risks and diminish profits for generic drug manufacturers.
Having received approval for a given process and location, the investment in developing new processes and waiting for new regulatory review would impose a burdensome and uneconomically wise cost. Other regulatory hurdles include: redefinition of the sampling plan, deviations that need to be handled differently, varying ways to control variability, the need for proper management, and the need to define a rationale for testing a continuous batch in comparison to traditional models.
Thus, regulators such as the US FDA and organizations like US Pharmacopoeia are focused on developing guidelines and standards for pharmaceutical CM that will give directions and help branded and generic drug manufacturers adopt CM in the best possible way. However, it is noted by many generic manufacturers that generic drug companies need to be able to produce drugs that were approved as continuously manufactured products via batch manufacturing. The paths to CM will be different for branded and generic drug companies.
BCC Research’s new report on Continuous Manufacturing for Pharmaceuticals (PHM214A) studied the market size of the CM market. The global CM market was valued at $2.3 billion in 2018, and is expected to grow at a CAGR of 8.8% through 2024 to reach $3.8 billion in 2024. While the highest market share was contributed by the branded drug companies and contract manufacturing companies together, generic drug companies are currently holding 5.6% of the total market share. The generic market is highly fragmented, with a majority of companies in the mid- to small-size segment. With less investment bandwidth and lower risk-taking potential, this segment has been a slow adopter of CM technology. With increasing support from regulators and visible long-term benefits of CM technology, generic drug manufacturers will follow suit. The market is expected to be driven by new technological enhancements and increased investments from generic companies, particularly in the Asia-Pacific region, where they are carrying out facility expansions. Companies such as Dr. Reddy’s Laboratories, Mylan Pharmaceuticals, and Aurobindo Pharma are developing continuous manufacturing lines in India. Some key developments in the CM market include:
-In December 2018, WuXi Biologics made an investment of $357 million in Dundalk, Ireland, to set up a new biologics manufacturing facility. The building will feature several single-use bioreactors for commercial biomanufacturing and is compatible with continuous bioprocessing. Additionally, the facility will boast 48,000-liter fed-batch and 6,000-liter perfusion bioreactor capacity bioreactors.
-In November 2018, Innovate UK awarded a grant of $1.85 million to a partnership of Pall Corp., Cell and Gene Therapy Catapult (an independent research and technology organization) and Cobra Biologics (a CDMO with a focus on the development of advanced therapy medicinal products). The collaborators are working to investigate CM of adeno-associated virus for gene therapy applications.
-In January 2018, SK Biotek inaugurated its new contract manufacturing facility in Swords, Dublin, Ireland, becoming the first Korean pharmaceutical company to invest in Ireland. SK Biotek had acquired this facility from Bristol Myers Squibb earlier in June 2017. The new facility will be used to manufacture pharma products to specification for other pharmaceutical companies on a contract basis.
-In December 2017, Fette Compacting and Glatt GmbH entered into a collaboration to develop an integrated solution for the continuous manufacturing of oral solid dosage forms. The new partnership combined the tablet compression expertise of Fette Compacting and the powder processing and tablet coatings skills of Glatt GmbH, hence providing an opportunity to co-develop CM technology for pharmaceutical manufacturers of OSD forms. As part of this partnership, the companies opened a new test facility in Pune, India, that offers customer trials for continuous direct compression and continuous wet granulation.
The FDA has been a strong proponent of CM and is encouraging pharma manufacturers to adopt CM. In February 2019, the US FDA made available the draft regulatory guidance for continuous manufacturing. Titled, Quality Considerations for Continuous Manufacturing, this draft guidance provides information regarding FDA’s current thinking on the quality considerations for continuous manufacturing of small molecule and solid oral drug products that are regulated by the CDER.
Despite some of the companies being early adopters, CM is still a technology that is being tested by a majority of the pharmaceutical industry. The FDA guidance and the ICH Q13 guidance are initiatives that are geared toward enhancing the adoption of CM by the pharma sector. Many proponents of CM technology, including GSK, Johnson and Johnson, Vertex Pharmaceuticals, and others are helping the agency to develop the right environment to foster the implementation of CM on a wider scale. However, organizations such as AAM and other generic pharmaceutical companies are treading with caution.
They want to ensure that the entry of CM does not prove anti-competitive to the pharmaceutical market and that the technology provides the promised benefits to manufacturers as well as to patients. This executive summary is based on the following market research report published by BCC Research: Continuous Manufacturing for Pharmaceuticals (PHM214A). For more information, visit https://www.bccresearch.com.
REFERENCE
- Schaber, S.D., Gerogiorgis, D.I., Ramachandran, R., Evans, J.M.B., Barton, P.I. and Trout, B.L., 2011.Economic analysis of integrated continuous and batch pharmaceutical manufacturing: A case study. Industrial and Engineering Chemistry Research 50: 10083-10092.
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Kamna Jhamb has has more than 13 years of research experience, of which more than 4 years have been devoted to market research. Before entering the field of market research, she worked as a Research Assistant in Lawrence Berkeley National Lab, CA. With a PhD in Microbial Technology, she has vast knowledge in the areas of microbiology, protein chemistry, and molecular biology. She has several international publications in reputed journals. For BCC Research, Dr. Kamna has written reports spanning many topics, including healthcare, finance, and biotechnology.
Total Page Views: 13866










