Issue:March 2023
CLINICAL TRIALS - New Technology & the Global COVID Pandemic Drive the Need for More Decentralized Trials
INTRODUCTION
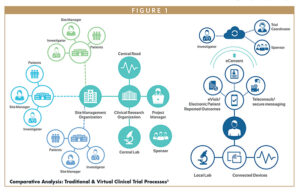
Remote clinical trials are becoming the new standard in clinical research. A variety of terms have been used to describe remote trials that incorporate patient-facing technologies, such as tablets, smartphone apps, or wearable sensors. They have been described as virtual trials, decentralized trials, remote trials, direct-to-patient trials, siteless trials, and hybrid trials. Whereas a digital trial is defined by the method used to capture the clinical trial data, a true digital clinical trial is one in which all data are captured without the use of any paper forms during the conduct of the study.
The COVID-19 pandemic has accelerated the ongoing shift to remote clinical trial monitoring. Remote site access and monitoring platforms are now an essential element of the clinical trial process and a vital connection between the sponsor, the clinical research organization (CRO), and the research site.
REMOTE CLINICAL TRIALS – KEY BENEFITS TO SPONSORS & PATIENTS
By digitizing the entire clinical trial process, remote clinical trial monitoring is becoming critically important to both sponsors and trial site administrators, offering the following benefits:
Complete access to remote clinical trial sites – By digitally connecting sponsor and trial site systems, the clinical trial administrator can securely access the site’s documentation from anywhere at any time. Sponsors and CRO monitors can securely access sites that they need to manage. This secured access allows monitors to complete their work remotely without onsite restrictions, saving considerable time and reducing overall cost.
Site and sponsor communication – The digitization of clinical trials allows for streamlined communication in the form of trial alerts and notifications, real-time dashboards, messaging, and email push notifications. These features improve communications and consolidate all conversations in which the work gets done – in the system itself.
Process compliance and trial oversight – The digitization of clinical trials creates a secure environment in which to comply with both HIPAA and 21 CFR Part 11 regulations. Built-in audit trails and the ability to view and verify trial documents provide a path to compliance. Adaptive data integration is used to integrate heterogeneous systems data. The ideal environment delivers “defense of depth” security, whereby security is controlled from a physical structure, such as entry to data centers, hardening networks, and servers. When it comes to application, implementing security at the user interface (UI), business, and data access layer component (DALC) levels enables data protection via concentric layers of security.
Automated workflows and repeatable standardized processes – With the digitization of clinical trial systems, the documents needed for submission to FDA and the workflow for review and approval can be automated. All trial documents are stored centrally and streamlined with secured access to all privileged users. With digitization, routing of documents is automated via a unified platform that allows administrators to easily redact, edit, and capture data as well as update versions of documents in a single database. The industry is moving toward a unified platform with a single database for all clinical trial products like electronic data capture (EDC), clinical trial management systems (CTMS), electronic trial master files (eTMFs), and electronic patient-reported outcomes (ePRO).
Easy tracking of study progress and trial sites – Digitization helps improve site performance and enables real-time tracking of study progress without site staff assistance. System alerts and notifications for document completion and other site activity can be easily set up for comprehensive tracking. Analytics with dashboards can provide a singular view of site efficiency, study timelines, document completion, and outstanding actions to manage trial activities more effectively.
THE EVOLUTION OF REMOTE CLINICAL TRIALS
Remote/virtual clinical trials have increased in popularity since the start of the COVID-19 pandemic, in response to the adaptations and the contingency plans that every industry, including the life sciences, have had to implement.
The technological advances made throughout the past 5 years have made remote clinical trials possible. With the technology tools available today, remote/virtual clinical trials bring clinical research directly into the participant’s home via a central and virtual coordinating site.
Remote clinical trials utilize devices that monitor and deliver vital information about patients. Wearables like Apple Watch, Fitbit, electrocardiogram (EKG) monitors, blood pressure monitors, and glucose monitors are also used to get a complete picture of the patient’s health.
Mobile health applications and telehealth technologies are used to collect medical data from participants and transmit this information to the central study center. Video conferencing has revolutionized the ability to remotely monitor and engage with patients and clinical trial managers. Clinical trial research companies believe the use of patient-facing technology helps in widening the pool of trial participants/volunteers, enhancing patient retention, improving data quality, and fostering a holistic, patient-centric experience. Patient-facing systems are designed to provide a wide range of computer- or internet-based services that support patient interactions with the healthcare system. Examples of these systems include patient portals, mobile applications, and online peer support communities.
The need for remote clinical trials is increasing because:
- Remote clinical trial solutions can significantly improve recruitment of patients and site staff, safety monitoring, patient education, pre-screening, and data verifications.
- Remote trials can deliver significant cost efficiencies to pharmaceutical and biotechnology companies, positively impacting their bottom lines.
- Implementation of decentralized and remote solutions also accelerates time-to-market for new drugs by reducing the overall length of the clinical trial life cycle. Other benefits include reduced enrollment periods, faster study initiations, lowered patient dropout rates, and improved data collection features.
- Remote visit set-ups can reduce the overall cost of conducting virtual clinical trials, compared to conventional clinical trial methods. Additional capital savings can result from the reduced number of operational clinical sites and lower patient travel reimbursements.
TECHNOLOGY IMPROVES THE EFFECTIVENESS OF REMOTE CLINICAL TRIALS
The growth of decentralized clinical trials is being enabled by several key technologies that span the entire clinical trial spectrum. Notable technology enablers include the following:
Patient-facing technologies – In remote clinical trials, patients need to be provided with choices related to accessing trial information, user-friendly training materials, video visits, and direct-to-patient study supplies, among other options. The availability of technologies that enable these choices can optimize a patient’s clinical trial experience, which is especially important to the overall success of any remote clinical trial.
Unified data platform – With the advent of data lakes and unified data platforms that support the entire life cycle of clinical trials, the effectiveness of decentralized trials has improved vastly. In decentralized trials, there is a variety of data sources, including devices, imaging technologies, electronic health records (EHRs), laboratory information systems (LIS), EDC, CTMS, and ePRO. Data platforms allow for real-time/remote access to all clinical trial data, and enable the deployment of automated and intelligent processes to provide near real-time data review. In addition, data platforms can facilitate the following:
- Real-time compliance monitoring to understand data completeness
- Data monitoring for safety
- Monitoring for data quality and completeness
On-site/remote consent from patients – Electronic automation of the patient consent process can enable flexibility and consistency in information exchange and data capture. The digitization of remote clinical trials provides easy-to-understand information and the ability to access consent information throughout a trial, ensuring appropriate regulatory compliance.
Clinical trial protocol development and design capabilities – The protocol is the playbook for any clinical trial. The advent of technologies that can connect patients remotely to trial sites can enhance the sponsor’s understanding of site and patient burden during protocol development and design, and can help determine the appropriate level of “decentralization” within a specific trial, increasing its overall probability of success.
ARTIFICIAL INTELLIGENCE ACCELERATES REMOTE CLINICAL TRIALS
In the past few years, technologies like artificial intelligence (AI), machine learning (ML), and natural language processing (NLP) have made an enormous impact across all aspects of clinical research.
Advanced analytics tools that use AI and ML are providing clinical trial administrators the power to make sense of the vast amounts of data collected through clinical trials. These tools enable advanced statistical predictive modeling and process automation to enhance overall study quality
Today, the volume, variety, and velocity of structured and unstructured data generated by clinical trials are outpacing traditional data management processes. The reality is that there is simply too much data coming from too many sources to be manageable by human teams alone. At Anju Software, we are constantly innovating tools, such as TrialMaster, an intuitive EDC suite that accelerates Phase 1-4 clinical trials and delivers features to drive decentralized trials. TrialMaster improves efficiencies and reduces workflow impact while enhancing data quality, resulting in faster study submission times.
As the remote clinical trial environment continues to evolve, AI/ML technologies show remarkable potential to automate data standardization while ensuring quality control, in turn easing the burden on researchers with minimal manual intervention.
The use of AI/ML-driven data management does more than accelerate data capture or provide generalized insights. These platforms seamlessly integrate large volumes of data from a breadth of sources and feature automation tools to streamline the review process. They can also clean and house all data in a single location and use custom ML algorithms to identify data errors, outliers, and false entries. As a subset of AI, NLP can identify site- or study-specific risks in both structured and unstructured data.
AI/ML technologies also enable predictive analytics that can maintain patient safety and improve site performance by detecting and mitigating potential safety issues. They can also facilitate site selection, more effective risk-based quality management, enhanced patient recruitment and engagement, and overall efficiency throughout the clinical trial life cycle.
AI and ML cannot solve every problem in clinical research. However, AI/ML tools can increase the amount of data collected, pull from multiple data sources, and ensure the “cleanliness” of data for researchers to properly analyze. In a few hours, these tools can accomplish tasks that would take human researchers months or even years. In short, as the industry leaps into new frontiers, AI/ML strategies are redefining the clinical development cycle like never before. With digital transformation leading the way, drug companies can redouble their efforts to get to the right therapies into the hands of patients faster, yielding advances that will revolutionize the space forever.
REFERENCES
- Yaakov, R.A., Güler, O., Mayhugh, T., Serena, T.E. Enhancing patient centricity and advancing innovation in clinical research with virtual randomized clinical trials (vRCTs). Diagnostics 11:151 (2021). https://doi.org/10.3390/diagnostics11020151.

Suhas Gudihal is Co-Founder, Anju Software, where he drives product strategy and direction as well as oversees Anju’s technology architecture and product development. Throughout his career, he has created and managed product engineering labs across various environments. He has successfully built and scaled up engineering teams for start-ups to large enterprises. He has successfully integrated over 20 acquired companies. He oversees Anju teams responsible for the development and commercialization of adaptive technologies for clinical trials, medical affairs, and data with world-class customer support. Leveraging AI and data-driven analytics, Anju’s leading suite of solutions, including TrialMaster, delivers data and application integration capabilities to the global pharmaceutical, biotech, and contract research Life Sciences markets. He earned his BS in EE, his MS in Computer Systems, and his MBA with specializations in High Technology and Innovation. He is also a Certified Management Accountant of the IMA, and a Fellow of Management Accountant institute of India.
Total Page Views: 3545