Issue:October 2018
CLINICAL TRIALS - How to Make Clinical Trials Patient Centric: Five Common Sense Steps
INTRODUCTION
Too many clinical trials fail because they can’t attract or retain enough patients. One recent study of 2,579 clinical trials showed 19% of them had been terminated for failing to recruit patients or for recruiting less than 85% of planned enrollment, seriously compromising the statistical validity of the results.1 Even trials that recruit enough subjects can flounder if too many of them drop out before the study is complete. For example, on average, one-third to one-half of patients enrolled in randomized controlled trials (RCTs) testing weight loss drugs quit early, within 1 year.2 One recent study testing a new therapy for patients with chronic pain due to spinal cord injury saw a 76% attrition rate, with only 8 of 46 patients who finished the study.3
Statistics like these have inspired pharmaceutical companies to try to make clinical trials more “patient centric” to better accommodate patients’ needs and preferences. This includes making it easier for patients to learn about and participate in trials, as well as improving the patient experience, not only to improve retention but to create future advocates for trial participation.
Research suggests the cumulative impact of a relatively modest investment in patient engagement avoids at least one protocol amendment, improves enrollment and adherence. Even more, retention in Phase III can produce a 500-fold increase in expected net present value of the investment.4 It can also shave up to 18 months off time to market.5
Most companies know they need to make trials more patient-friendly, but few have access to the tools and methodologies to transform their protocol design process. Fortunately, thoughtfully applied common sense practices can create better patient experiences and more robust trials.

FIVE STEPS TO OPTIMIZE STUDY PROTOCOLS FOR PATIENTS
To create a patient-centric trial, design optimization should be verified via systematic processes before the trial starts. To test and refine protocols to achieve the right balance between scientific rigor (ie, generating sound and comprehensive data to demonstrate safety and efficacy) and feasibility for patients (ie, designing a trial within the logistical and practical reach of the maximum number of people), companies can use the following five tools:
No. 1 – Create an Accurate Profile of the Target Patient
Researchers spend months establishing eligibility criteria for clinical trial participants to ensure a study can answer precise scientific questions. To make a trial patient centric, it’s also necessary to dedicate sufficient time to understanding patients’ everyday lives and the parameters that affect their participation.
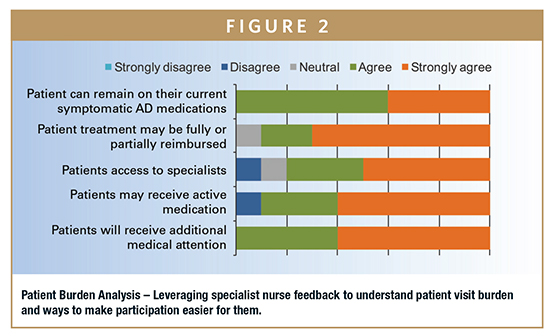
Having a detailed profile of a trial’s patient population (and understanding who they are as individuals) can inspire improvements to the protocol that can make the difference between success and failure. Key issues that must be addressed include:
-Demographics of the patients in the study and how that may impact their habits. Are they elderly and may have difficulty leaving home? Are they young adults who can travel easily? Or, are they small children who must be supervised by parents?
-Type of treatment being tested may impact motivation. For example, is it curative or palliative?
-Payment. Will the costs of participating in the trial be reimbursed?
-Real life impact. What is life like with this disease? Do patients have routine, frequent doctor appointments and/or hospitalizations, similar to trial site visits or interventions? Or do they have very few medical interventions, making trial-related testing and procedures more out of the ordinary?
An accurate patient profile is a good starting point when shaping trial design, providing an opportunity to explore what has the biggest impact on participants. For example, if given the option, how would they weigh up a choice between a lower visit burden or fewer invasive procedures? By profiling the patient, we can begin to explore such sensitive areas to create a truly patient-centric approach. We’re shifting the discussion from purely answering the scientific question of “Does this patient fit the eligibility criteria?” to “How would the practicalities of study participation impact their decision to join and remain in a trial? And how can we minimize the geographical, financial, and practical barriers to participation?”
No. 2. – Survey Patients & Caregivers for Insights
Pharmaceutical companies can survey patients, caregivers, and nurses/physicians about key aspects of a study to gauge how it may be received. Eliciting such feedback seems like common sense, but incorporating patient and caregiver needs, views, and experience is a relatively new approach in this context — and one that has shown great value.
For example, one company designing a study in young children with severe epilepsy worried that the three 72-hour hospital visits required in the protocol might take too high a toll on very young patients. They surveyed caregivers to find out, on average, how many times in a normal year their children were admitted to the hospital and for how long. The results were surprising. Parents said their children wound up in a hospital about three to five times per year on a routine basis — and that most visits lasted at least 3 days. The company concluded that the trial protocol did not place an undue burden on these already very sick patients and their families as the visit burden was similar to standard of care. Because the required hospital visits were needed to conduct a critical test, the company followed the protocol as planned. But the survey uncovered a different problem: parents reported that caring for their other, healthy children was the biggest challenge of epilepsy-related hospitalizations. To address that, the company explored plans to work with sites to offer flexible visit timing to simplify for parents the process of securing childcare.

No. 3 – Seek an Unfiltered Reality Check on the Web
There has been an explosion of open online forums, tweets, and blogs where patients share stories about their disease and treatment options. “Listening” to the web through search terms, and dipping into online chatter can give companies unfiltered insight into what really matters to patients. Patient stories capture, often poignantly, what it’s like to live with a disease in nonmedical terms and may inspire new approaches and ideas.
For example, one company developing a treatment for Crohn’s Disease read a number of blogs written by the children of Crohn’s sufferers and got a deeper perspective on how frightening the disease can appear to them. Crohn’s patients can become alarmingly thin and weak, and their children may worry that their parent will not survive. If their parent is hospitalized, some stay with relatives for extended periods, which can cause fear and upset. The high volume of stories from the children and caregivers of Crohn’s patients online indicated that the whole family is highly involved in treatment decisions. Therefore, to optimize recruitment, the company tailored its educational materials to help facilitate a joint decision-making process around trial participation.
In a recent Alzheimer’s Disease (AD) study, an analysis of web postings revealed that far more online chatter about AD came from caregivers than from patients. Therefore, an important component of the recruitment and retention strategy was to engage and educate caregivers by explaining the objectives of the study to them and treating them as partners. The web analysis also revealed that AD patient advocacy groups (PAGs) are very active online and are a trusted source of information and support for caregivers and patients. The company thus knew it would need to leverage existing relationships with PAGs, as well as forge new ones, in addition to high online visibility to meet its recruitment goals.
No. 4 – First Quantify, Then Mitigate, the Study Burdens
Most patients have never participated previously in a clinical trial. Although they may know a lot about living with their disease, they are inexperienced in planning clinical research logistics. So, while you can learn a lot from patients, you need to probe further.
Nurse specialists with deep experience at high-volume/high-performing investigative sites are among the most astute judges of the burdens a clinical trial can impose on patients. Their analysis of the potential benefits and challenges of study participation is a valuable resource. Questions they can answer include the following:
-How does the burden of this study compare to the standard of care?
-What could be changed to make it easier for patients?
-How long will each visit last?
-What information will be most useful to support patients in learning about and participating in this study?
Recently, a team of nurse specialists drawn from a network of top research sites helped make a protocol more patient friendly by nixing several planned patient questionnaires. Looking at the number, length, and scope of these questionnaires, the nurses predicted patients would balk at answering so many questions so often during the study. In this case, the solution was to prune some of the trial’s exploratory endpoints, which cut down on the number and length of the questionnaires.
However, even experienced clinical staff can misunderstand the issues that worry or, conversely, motivate a patient. For example, in a recent osteoarthritis study, a large majority of experienced nurses who were surveyed about the trial’s design said that the inclusion of home nursing visits in a protocol (versus visits at the investigative site only) would have no impact on a patient’s willingness to participate in a study. In contrast, a patient survey asking the same question showed patients viewed home visits as a positive benefit. The survey didn’t capture enough data to explain the reasons for this discrepancy, but it is possible the nurses’ responses were based on how attractive home visits were from their perspective as caregivers rather than from the perspective of a patient.
No. 5 – Simplify & Standardize Informed Consent
A typical informed consent form is notoriously long and can include a lot of terminology that is unfamiliar and, to most, incomprehensible. Recruitment and retention can be improved by simplifying the informed consent process and making the core document more comprehensible. One way to communicate complex information simply is to break the elements of a study into short (no more than 1 minute) video modules.
For example, a recent trial enrolling 5- to 11-year olds in the United States and South Africa had a complex schedule of events, including two mandatory overnight stays in the hospital. Patients and their parents needed a clear explanation of the trial’s procedures and expectations to provide informed consent. So, the pharmaceutical sponsor developed an animated video (translated into the local languages) that explained the study’s activities, and showed how the drug worked. By providing the information in this way, parents and children gained a good understanding of what study participation would mean for them. With this tool, enrollment for this trial completed 5 months ahead of schedule with no dropouts.
PATIENT CENTRICITY IS NOT A BUZZWORD
Optimizing a trial protocol for patients is always preferable and, with a bit of care and thought, most often achievable within the normal trial planning timeline. And patient centricity is achieved not in isolation of the doctors and nurses who routinely care for the patients but rather in collaboration with them. Investing care and thought can yield big rewards. It also may prompt positive changes to a protocol, or validate the existing plan. In either case, elevated recruitment and retention levels, and happier trial participants, is worth the investment.
REFERENCES
- Carlisle B, Kimmelman J, Ramsay T, MacKinnon N. Unsuccessful trial accrual and human subjects protections: An empirical analysis of recently closed trials. Clin Trials. 2015;12(1):77-83.
- Delahanty L, Riggs M, Klioze S, Chew R, England R, Digenio A. Maximizing retention in long-term clinical trials of a weight loss agent: use of a dietitian support team. Obesity Science & Practice. 2016;2(3):256-265. doi:10.1002/osp4.57.
- Carvalho S, Leite J, Jones F, Morse LR, Zafonte R, Fregni F. Study adherence in a tDCS longitudinal clinical trial with people with spinal cord injury. Spinal Cord. 2017 Dec 13. doi: 10.1038/s41393-017-0023-5. [Epub ahead of print]
- Levitan B, Getz K, Eisenstein E et al. Assessing the Financial Value of Patient Engagement. Therapeutic Innovation & Regulatory Science. 2017:216847901771671. doi:10.1177/2168479017716715.
- Ibid.
To view this issue and all back issues online, please visit www.drug-dev.com.

Rosamund Round is PAREXEL’s Patient Centricity Lead and spends her time devoted to simplifying the patient journey in clinical trials. Focused on the reduction of geographical, financial, and practical barriers to study participation, she is excited by the industry shift toward a truly patient-centric approach. Her first job in an oncology clinic at Massachusetts General Hospital sparked her passion for putting patients at the center of clinical research planning and implementation. Subsequent roles in patient recruitment in both the pharma and CRO industries have enabled her to innovate and explore better ways to communicate with patients. This includes addressing literacy and health literacy, exploring technological advancements, and constantly scanning the environment to help generate new ideas to make clinical trial participation more accessible and convenient for patients.
Total Page Views: 7361










