Issue:March 2024
CLINICAL TRIALS - Digital Endpoint Integration in Clinical Trials: Key Considerations & Nuances Per Therapeutic Focus
INTRODUCTION
In today’s clinical trials, industry stakeholders of all kinds are embracing the use of digital health technologies (DHTs), such as medical-grade sensors and wearables, to collect respiratory rate, oxygen saturation, blood pressure, and other physiological parameters directly from trial participants in the comfort of their homes or wherever they live or work.
The popularity of this data collection modality is unprecedented. Patients have more flexibility to participate in trials and improved experiences while providing trial sponsors with richer insights captured in near real-time to guide smarter decision-making. The number of trials integrating DHTs across therapeutic areas is growing by the day. With regulatory support and guidance, these tools are allowing sponsors to include patients who potentially could not be in trials due to geographical and logistical barriers. They also evaluate diseases and related patient experiences in near real-time with continuous data collection and review opportunities.
However, when considering incorporating digital endpoints into trial design, it is essential sponsors, their CRO partners, and site teams account for the key clinical and operational aspects discussed further, which can impact how well clinically meaningful outcomes are captured and analyzed. This includes planning to ensure patient safety and data quality are not compromised. Additionally, therapeutic needs can vary greatly among patient populations and conditions being studied. Taking those nuances into consideration is critical to optimizing trials with digital endpoints.
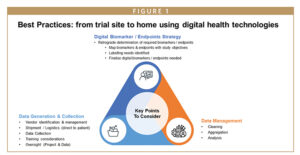
KEY COMPONENTS FOR DHT INTEGRATION
Before all else, sponsors and CROs need to ensure the right mix of experts is engaged in the trial design process and give the following careful thought:
- Protocol/program-specific digital endpoint strategy
- Data generation and collection
- Data management
PROTOCOL/PROGRAM-SPECIFIC DIGITAL ENDPOINT STRATEGY
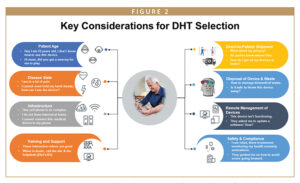
One of the first critical steps toward trial execution effectively using DHTs is the selection of the device(s) and making sure they are fit-for-purpose to program objectives and endpoints. In the ever-evolving drug development landscape, there are numerous devices capable of collecting and providing similar physiological parameters. Therefore, best practice requires matching the study objectives with digital endpoints that can prove the hypothesis as per study design and include the specific functionalities that can generate those specific digital endpoints.
Additionally, leveraging therapeutic experts’ insights during trial planning can help determine what device(s) may work best for specific patient populations. For example, using impulse oscillometry technique or cough monitors for respiratory diseases may seem attractive. However, we must be aware of the regulatory acceptance of the data, given most data is under research, and currently, no acceptable digital endpoint exists. In this case, sponsors of respiratory disease trials are advised to rely on established spirometry data for pulmonary function testing but to keep up with newer technologies coming to market so they may be used, as appropriate, when acceptable digital endpoints are available.
Another example is choosing devices that have blood pressure cuffs appropriate for a wide range of arm circumferences possible in various patient populations. Blood pressure readings can be grossly erroneous if inappropriate cuff sizes are used.
Further, deriving the target quantity of data needed along with the timeframe and frequency of data collection is imperative for both the digital endpoint strategy and labelling needs for marketing.
MANUFACTURER CHECKLIST
When the endpoint strategy is finalized, the risk-based qualification of the device manufacturer is the next step in the journey. Sponsors should consider the checklist below to ensure their partner manufacturer can fulfill end-to-end needs for the trial’s duration:
- Review the manufacturer’s data security, privacy, scalability capabilities, and financial standing. This includes knowing whether local to global reach and services are available, including device shipping to and from sites and patients.
- Ability of its team to participate in training in appropriate disposal of devices and their parts (eg, batteries) or medical waste. It is also key for patients and sites to be trained in this for process compliance.
- Provision of mobile phones and laptops along with internet connectivity and managing devices remotely, including remote technical troubleshooting and coordinating software updates.
Specifically, regarding device shipping and logistics, while connectivity for individuals worldwide is much improved in recent years, making sure medical devices reach end users as planned and within timelines in multiple countries has its own challenges. Among other issues, this means accounting for variations in customs, import requirements, device approvals, and appropriate labelling of devices.
The sponsor’s shipment oversight partner will need to have strong and detailed global kitting and supplying/re-supplying capabilities to meet start-up timelines and needs throughout the trial. Consolidating all necessary materials, such as investigational product(s), device(s), and training instructions and content, within one or few shipments can improve the patient experience and burden.
EFFECTIVE DATA GENERATION & COLLECTION
Given the continuous data monitoring and collection coming in from DHTs, sponsors can gather numerous insights from one device. When considering hundreds to thousands of patients sharing data via a DHT in one study, sponsors have potential to gather millions of data points. This can be an exciting opportunity for diving deep into patient experiences. However, there is an acute need to balance actions between digital data collection and ensuring patient safety remotely, and again, obtaining adequate data quantity to meet the study objectives.
The main factors affecting successful and quality data collection without compromising patient safety include the following:
Purposeful DHT Integration: While there are many devices in use that have endpoints cleared in one or two countries, as the industry strengthens its commitment to improving access to trials to traditional underserved patient populations globally, closely evaluating the device from a patient’s perspective will be critical. This includes gauging ease of use, local language capabilities, accommodating internet connectivity challenges and more.
Ease of Data Transfer: Along with ease of use, ensuring the device design allows patients to transfer data to central software or database with minimum effort will be beneficial to collecting insights as needed. As device functions are interactive and straightforward, the likelihood of securing adequate quality data will increase.
Enhanced Passive Data Collection: To further reduce patient burden, a shift to passive data collection can be critical. It is vital to think about trial participants’ physical and mental condition, ages and ability to comprehend the requirements of data collection.
For example, if participating in an oncology study in which enrolled patients’ life expectancy is less than 1 year, sponsors and CRO partners must consider the patients’ perspective and emotional mindset before adopting a DHT that requires the patient to manually collect multiple spirometry records on a regular basis. Or, if considering pediatric patients, explaining the appropriate spirometry device usage and technique(s) and ensuring children follow instructions can be difficult, so reduced compliance is a distinct potential. For children, alternative technologies, such as forced-oscillation technique, which requires the young participant to breathe normally, should be considered. While this is attractive, the outputs are yet to be cleared from a regulatory perspective. The technology is being widely used in clinical practice to facilitate better insights for physicians; however, use in clinical trials for primary or secondary endpoints is approximately a year or more away.
Looking ahead, sponsors and CRO partners are moving toward creating device-agnostic software with integration capabilities that enable electronic source (eSource). This capability can help eliminate manual data entry and transcription and traditional methods of source data verification. It can also help reduce administrative burdens on site teams and patients, who will not have to remember multiple usernames and passwords to access DHT software.
TRAINING & SUPPORT
Another integral component to an effective digital endpoint strategy is adequate training and support services to ensure proper DHT use and data collection. Some key aspects to consider include the following:
- Training will need to be timely and as early in the process as possible but not so early that the end user forgets it. Challenges during use and execution are to be expected but timing of problems cannot be anticipated.
- Tailored and adequate training materials should be made available to users anytime via the applications and other systems.
- Performing psychometric analysis of the material provided can help gauge how well the end users understand what is needed of them and the importance of being compliant. This is still a new and evolving methodology but has demonstrated benefits to sponsors.
- Efficient 24/7/365 local language support to sites and patients. Having a support team for patients must be the collective goal of the sponsor, CRO partner and site team. However, this is not without challenges.
For example, the US FDA’s guidance for assessing pressor effect, which helps gauge how much blood pressure is increased due to treatment, notes that cardiovascular risk evaluation of any drug administered for more than 12 weeks is imperative. During execution, recording ambulatory blood pressure data for a finite period or during visits is important, and the devices are blinded to the subjects. The variance window to repeat these measurements is also limited. This means efficient collection of requisite data must be conducted correctly the first time, and as such, training and ongoing support for patients and site teams is necessary. Appropriate education may include hands-on training and simulations in front of expert technicians, easy-to-digest instructional videos, and other assets. This can help participants feel comfortable using the device on their own and ensure any potential stress is curbed to not alter blood pressure measurements. On the other side, making sure clinicians are equipped with near real-time feedback through compliance reports upon data upload helps ensure details about patient errors are promptly addressed with re-training, etc.
MANAGING PATIENT SAFETY
As we transition from sites to patients’ homes, monitoring patient safety and medical management is a challenge and a new opportunity. While data can be made available in near real-time, sponsors and study teams have an opportunity to better manage the patient’s condition that was not available prior to DHTs. However, it is vital sponsors and CRO partners thoroughly walk-through concerns regarding data privacy, making sure patients’ identifiable information is secure per regulatory requirements and Good Clinical Practice guidelines.
Developing key metrics early in trial planning and design can help ensure adequate support for patients, including asking such questions as:
- What is the threshold of acceptable data quantity?
- What are specific parameter ranges that should trigger a patient health review either remotely or in person?
- What are the appropriate compliance metrics to guide study teams and help identify re-training needs for patients based on trends of data collected during the trial?
HOLISTIC DATA MANAGEMENT
It is not physically feasible to manually perform such tasks as data cleaning, aggregation, and analysis effectively in trials with digital endpoints, given the vast amount of data being collected. As such, sponsors may consider the tips below for a holistic approach to data management and oversight:
Data Cleaning: Build in automatic edit checks at multiple levels to receive clean data right from the beginning and include:
- Data entry by site (for first time), and thereafter, patient from their home.
- Demographic discrepancy edit checks (per device used). For example, edit checks for absurd values from devices being transferred, subject’s age, gender, race, etc., that need to be collected as per the device needs:
-For electrocardiogram data, age and gender are key.
-For blood pressure data, age, gender and dominant hand are key.
-For spirometry data, age, gender and environmental factors are key.
-Provision to share immediate feedback to patients and site teams on compliance (data quantity and quality) upon data upload.
Data Aggregation: Time-synchronized data aggregation for patients using multiple devices and across different geographies can be leveraged to derive succinct data covering evaluation and event management according to time zones and daylight savings time. Data conversion into a standardized format compatible with downstream needs related to derive meaningful outcomes is an important and complex aspect to consider. For example, this is key when there is a need to combine the dose administration time with before and after spirometry data. Accurately collecting and combining data from multiple sources is extremely important.
Data Analysis: Lastly, statistical analysis that is capable of proving the study hypothesis and outcome is essential. This can run smoothly if the data pertaining to the objectives is accurate and complete. For use cases related to algorithm development or using an existing algorithm for the first time on a compound, interim evaluation of effectiveness of developed algorithms is necessary to identify issues early and either course correct or shelve the project with limited loss.
SUMMARY
As previously noted, enhancing the patient experience and reducing burden is key in today’s drug development landscape, and DHTs can certainly play a positive role in that. However, there is quite a lot for sponsors and related stakeholders to consider when determining whether it may be beneficial to integrate DHTs into trial design to better meet the needs of the specific patient population while maintaining their safety and upholding data integrity. When thoroughly considering varying nuances for optimal trial design with digital endpoints, it is possible to successfully plan for and execute clinical trials using DHTs, allowing sponsors to make more informed decisions for the patients they aim to serve.

Tapan Raval is the Director, Medical Devices Science and Technology, Strategic Solutions, Connected Devices at IQVIA. With a medical and clinical research education background, he has nearly 2 decades of experience working in the CRO industry. He has helped oversee centralized cardiac safety operations and data management as well as create end-to-end solutions for using data generated by medical devices as part of clinical trial safety and efficacy end points.

Dr. Ganesh Gundi is the Director, Medical and Lab Operations Connected Devices at IQVIA. He offers more than 15 years of experience with IQVIA, managing clinical trials that have supported more than 150 customers primarily with ECG, Holter, patches, telemetry services, and medical monitoring.
Total Page Views: 4612