Issue:June 2020
CHIMERIC COMPOUNDS - “Dopastatins”: One Molecule Targeting Two Receptors for the Treatment of Pituitary Tumors
THE PITUITARY GLAND
The pituitary gland sits near the base of the brain and is responsible for the production of many different hormones, chemical messengers that circulate through the bloodstream to carry signals throughout the body. The pituitary gland is sometimes referred to as the master gland because it plays an important role in regulating multiple pathways and processes in the human body. For example, certain cells in the pituitary gland produce growth hormone (GH), which regulates growth and tissue repair throughout the body. Other examples include the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), two hormones key to reproduction and the development of sexual characteristics. Hormone production by the pituitary is continuously regulated by chemical signals from the brain, and by feedback from the target organs throughout the body.
AN INTRODUCTION TO PITUITARY ADENOMAS
Pituitary adenomas are tumors that arise from and grow within the pituitary gland. These tumors can be divided into two categories based on size: micro- or macroadenomas. Microadenomas are smaller than one centimeter, whereas macroadenomas are larger.
FUNCTIONING VERSUS NON-FUNCTIONING PITUITARY ADENOMAS
Pituitary adenomas are also classified as “functioning” or “non-functioning.”1 If pituitary adenomas produce active hormones, they are classified as “functioning,” and result in diseases caused by hormonal excess. In addition, the growing tumor can cause symptoms due to pressure on surrounding structures (brain, optic nerves, normal pituitary tissue, etc). Other pituitary adenomas are “non-functioning,” meaning they only secrete inactive hormone fragments and thus don’t affect the normal processes in the human body.2 Despite the lack of active hormone production, non-functioning adenomas are still dangerous due to their size. In fact, they can be inherently harder to treat than functioning adenomas. Because they generally do not cause symptoms while the tumor is small in size, they can remain undetected for extended periods. Consequently, at the time of diagnosis, these tumors are typically very large. They may also invade nearby critical brain structures, such as the optic nerve or other nerves and important blood vessels, leading to debilitating symptoms, including vision loss, severe headaches, loss of pituitary function, and diminished quality of life for a patient.2 While the initial therapy for both types of pituitary tumors is usually some form of neurosurgery, in the majority of cases, surgery fails to completely eliminate the tumor. If the tumor is functional, surgery often fails to control the associated disease. Subsequent treatments aim to control excess hormone secretion (in functional tumors) and may be combined with repeated surgeries and radiation therapy to control tumor growth, which can result in permanent damage to surrounding structures. Consequently, efforts are under way to develop a drug therapy to shrink or stabilize pituitary tumors.
TWO IMPORTANT RECEPTORS: SOMATOSTATIN & DOPAMINE
It has been established that receptors for the hormones dopamine, and somatostatin, which are natural regulators of pituitary function, are expressed at varying levels by many pituitary tumors. These hormones can also play an important role in tumor suppression and have been a target of interest for drug development for several decades. Most drug development efforts have focused on controlling hormone production by a functioning pituitary adenoma. One of the most studied diseases is acromegaly, a condition resulting from excess GH secretion.
A CLOSER LOOK AT ACROMEGALY
Patients with acromegaly produce an excess of GH, which in turn causes excess production of a hormone called insulin-like growth factor (IGF-1). When the disease occurs in children prior to puberty, the excess IGF-1 causes accelerated growth and abnormally tall stature, referred to as gigantism. In adults, the excess IGF-1 results in enlargement of the jaw and skull, bones, soft tissues, and certain organs. If left untreated, the excessive tissue enlargement may lead to serious life-threatening complications.3,4
It is now well-established that somatostatin suppresses GH secretion by acting through two different somatostatin receptor subtypes, SSTR2 and SSTR5. The pituitary adenomas that lead to acromegaly consistently express high levels of both. Given this, long-acting somatostatin analogues like octreotide and lanreotide have become accepted treatments for controlling GH secretion in patients with acromegaly.5
However, these widely used somatostatin analogs are only fully effective in normalizing GH and IGF-1 in roughly 35% of patients.6 This is due to the fact that lanreotide and octreotide were developed prior to the identification of the somatostatin receptor subtypes, and before knowledge of the receptor subtypes involved in suppressing GH secretion in humans. Consequently, the currently available clinically used analogs of somatostatin are highly potent at SSTR2, but have only limited activity at SSTR5, which is now known to play an important role in suppressing GH secretion in humans.6
Developing an agent with high potency at both SSTR2 and SSTR5 demonstrated that combined activation of both SSTR2 and SSTR5 results in much greater efficacy in suppressing of GH; however, it was discovered that SSTR5 activation also directly suppresses insulin secretion from the pancreas and results in hyperglycemia. This negative side effect has limited the development of therapies that are highly active at SSTR5. Nevertheless, these studies established the concept that activating multiple receptors may offer enhanced therapeutic options for pituitary tumors, including those that cause acromegaly as well as other endocrine diseases.
A CLOSER LOOK AT PROLACTINOMAS
Another important regulator of pituitary function is dopamine. Among other roles, dopamine normally inhibits the secretion of prolactin, a hormone with many functions, including milk production in women after childbirth. A prolactinoma is a non-cancerous pituitary tumor that secretes excess amounts of prolactin, which can cause dysregulated breast milk production, reduced sex drive, and impaired reproductive function in affected individuals. Pre-menopausal women may also experience irregular menstrual cycles.7 Prolactinomas are the most common pituitary tumors, with a prevalence ranging from 0.3 to 0.5 per 1,000 in the general population.8
Because prolactinomas express a subtype of dopamine receptor known as D2R, dopamine agonists specific to this receptor are widely used to treat these tumors.9 Dopamine agonists selective for D2R, such as cabergoline, are also sometimes used to treat other pituitary adenoma types.10
A COMBINATION APPROACH
As somatostatin and dopamine-based medical treatments for acromegaly and prolactinomas became available, it was eventually demonstrated that a combination of somatostatin and dopamine receptor agonists results in greater control of GH and IGF-1 in acromegalic patients than the use of either agent individually.11 These observations led to the idea of developing a single chimeric compound that interacts with both somatostatin and dopamine receptors (Figure 1).12 When tested directly on tumor cells from acromegalic patients, the combination of individual somatostatin and dopamine analogs was no more effective in suppressing GH secretion than either agent alone. However, when the somatostatin and dopamine activities were present in the same, single compound, there was a tremendous increase in both potency and efficacy in suppressing GH secretion. Through additional studies, it was determined that the optimal chimeric drug compound should have potent SSTR2 and D2R activity with moderate activity at SSTR5, given the negative side effects observed with potent SSTR5 activation.12
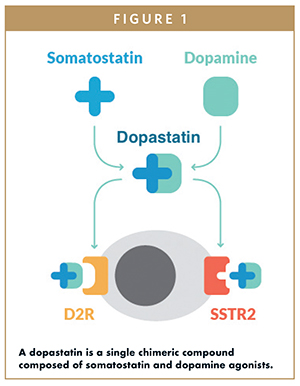
USE OF SOMATOSTATIN-DOPAMINE CHIMERIC COMPOUNDS FOR THE TREATMENT OF ACROMEGALY
While an actual mechanism for the enhanced efficacy of the chimeric compound versus the use of individual agonists remains elusive, it has been hypothesized that somatostatin and dopamine receptors interact to form heterodimers (new receptors formed from the physical joining of the somatostatin and dopamine receptors) that lead to a greater effect when activated in comparison with activation of the individual receptors.6
Arising from optimization studies that tested a number of different chimeric compounds, the first lead chimeric compound identified was BIM-23A760 (now TBR-760), which demonstrates greatly enhanced potency and efficacy in suppressing GH and prolactin from cultured human pituitary cells collected from acromegaly patients who were resistant to conventional somatostatin analogue therapy. TBR-760 has also been tested in healthy primates and demonstrated to induce a dose-dependent suppression of GH and prolactin, without affecting either insulin secretion or glycemic control.
Human clinical trials demonstrated that TBR-760 induces prolonged suppression of prolactin levels in healthy individuals and suppression of GH in acromegaly patients without significant side effects.12 When administered chronically, however, the compound’s efficacy decreased over time. To investigate why, researchers analyzed patients’ blood samples for major breakdown products (metabolites) in the body. They found a major metabolite with high affinity for the dopamine receptor that had a significantly longer duration in the blood than the parent compound, TBR-760. It is believed that the metabolite gradually accumulates and may bind to available dopamine receptors, reducing the number of available dopamine receptors available to bind to TBR-760. This, in turn, may have reduced the interaction of TBR-760 with somatostatin-dopamine heterodimers; however, TBR-760 was still able to bind to somatostatin receptors and thus still had some impact on GH. The result was that the added potency observed in vitro that is attributed to the effect of the chimeric was dampened.6,13
Nonetheless, the concept of a chimeric dopastatin compound as a treatment for acromegaly was demonstrated to be valid, and additional work exploring applicability and potential use with other pituitary tumors is well under way. Of particular interest is the use of TBR-760 as potential treatment for another pituitary tumor, non-functioning pituitary adenomas (NFPAs), in which the formation of the metabolite with potent dopamine activity could be a major advantage. If one considers the receptor ratio of relatively equal levels of dopamine and somatostatin receptors in acromegaly versus NFPA, the high level of dopamine and lower level of somatostatin receptor found in NFPA is well-suited to TBR-760 and its potent dopaminergic metabolite.11,13 The metabolite will bind to dopamine receptors, but with the high level of expression, there will still be dopamine receptors available to allow TBR-760 to bind, as well as allowing the potential for it to interact with somatostatin-dopamine heterodimers and realize the added potency.
NON-FUNCTIONING PITUITARY ADENOMAS (NFPAs)
NFPAs only secrete biologically inactive subunits of hormones, so they do not produce any associated hormone excess syndrome. Because of this, NFPAs often grow very large by the time of diagnosis and may lead to debilitating symptoms if left untreated.14 For example, the pituitary gland sits close to the optic nerves that connect the brain to the eyes. A growing NFPA can put pressure on these nerves, causing patients to experience vision loss. Additionally, because the pituitary is also situated near branches of the carotid artery, a key vessel that provides blood to the brain, pressure on the carotid arteries can compromise the blood supply of the brain. Other symptoms associated with NFPAs include intractable headaches from pressure on the brain and hormone deficiencies from crushing the surrounding normal pituitary tissue.13
Without available drug therapy, transsphenoidal surgery (TSS), an invasive type of neurosurgery to remove or reduce the tumor mass, remains the mainstay of treatment. While effective in rapidly relieving pressure on the surrounding structures, patients often experience regrowth of the tumor, which occurs in about 50% of cases as a result of residual tumor.13 When regrowth occurs and threatens surrounding structures, at present, the only options available are repeated transsphenoidal surgery and/or radiation therapy, both of which significantly increase morbidities, such as the risk of hormone deficiencies, and there is even a small risk of mortality from radiation therapy. In addition, radiation therapy significantly increases the risk of stroke and neurocognitive dysfunction. Magnetic resonance imaging (MRI) scans are routinely used to monitor tumor recurrence and tumor growth. The need for continued monitoring and the risk of related disease complications creates a lifelong psychological and financial burden for both patients and caregivers.
A drug therapy that is able to shrink or stabilize NFPAs and that has the potential to prevent the need for surgery or radiation would be a welcomed, radical treatment paradigm change for patients with NFPA, their families, and physicians.12
USE OF SOMATOSTATINDOPAMINE CHIMERIC COMPOUNDS FOR THE TREATMENT OF NFPAs
NFPAs consistently express very high levels of dopamine receptors and express variable levels of somatostatin receptors.12 Just as the enhanced efficacy of activating both receptors was demonstrated in acromegaly patients, this type of approach is also being considered as a therapeutic option for NFPAs. Although the chimeric dopastatin did not prove to be more effective than somatostatin analogs alone in acromegaly patients due to the formation of a potent metabolite that bound to dopamine receptors, it is hypothesized that the much higher ratio of dopamine receptors versus somatostatin receptors in NFPAs will allow both dopastatin and its metabolite to realize their full therapeutic benefit, by acting through both the dopamine and somatostatin pathways.
TBR-760 has been shown to suppress the growth of primary cultures of human NFPA cells.12 In addition, in a mouse model that develops highly aggressive NFPAs, treatment with TBR-760 has been demonstrated to completely arrest tumor growth. These findings provide reason to believe that chimeric dopastatins, and specifically TBR-760, may represent the first effective drug therapy for the treatment of NFPAs.
LOOKING FORWARD
To date, there are only limited, invasive, and high-risk treatment options available for patients with NFPAs. Even after neurosurgery and radiation, many of these tumors recur. The need for continued monitoring and risk of developing complications also creates a significant burden for patients and caregivers and leads to increased healthcare costs.
Additional clinical studies are needed to determine the extent of tumor shrinkage or stabilization possible using dopastatins, and, ultimately, to understand whether this therapeutic approach can impact these tumors and potentially change the treatment paradigm. A Phase 2 clinical trial investigating the use of TBR-760 in NFPA patients is set to launch this coming year.
Dopastatins with different properties may also prove effective for the treatment of acromegaly or prolactinomas. Developing a compound with receptor affinities that closely match the receptor profile of the specific tumor type will be key in this endeavor.
Ultimately, this novel class of compounds represents a potential treatment for neuroendocrine tumors that are vulnerable to the synergistic dual activation of somatostatin and dopamine receptors. The potential to replace repeated surgeries, radiation therapy, and even first-line, initial surgery with a drug therapy that shrinks or stabilizes tumors is incredibly attractive.
REFERENCES
- What Are Pituitary Tumors? Retrieved from https://www.cancer.org/cancer/pituitarytumors/about/what-is-pituitary-tumor.html.
- Batista RL, Musolino NRC, Cescato VAS, et al. Cabergoline in the Management of Residual Nonfunctioning Pituitary Adenoma: A Single-Center, Open-Label, 2-Year Randomized Clinical Trial. Am. J. Clin. Oncol. 2019 Feb;42(2):221-227.
- Carmichael JD, Bonert VS, Nuño M, et al. Acromegaly clinical trial methodology impact on reported biochemical efficacy rates of somatostatin receptor ligand treatments: a meta-analysis. J. Clin. Endocrinol. Metab. 2014 May;99(5):1825-1833.
- Acromegaly. Retrieved from https://rarediseases.org/rare-diseases/acromegaly/.
- Taboada GF, Lugue RM, Bastos W, et al. Quantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and nonfunctioning pituitary adenomas. Eur. J. Endocrinol. 2007 Jan;156(1):65-74. .
- Hill J, Tsomaia N, Kim S, Dong J, Culler MD. Chimeric Somatostatin-Dopamine Compounds (Dopastatins) for the Treatment of Neuroendocrine Disease. 2016; Comprehensive Medicinal Chemistry
- Dollar JR, Blackwell RE. Diagnosis and management of prolactinomas. Cancer and Metastasis Reviews. 1986; 5(2):125-138.
- Maiter D. Current Challenges in the Management of Prolactinomas. 2014 Meet-The-Professor: Endocrine Case Management,170-174. doi:10.1210/mtp3.9781936704835.ch37.
- Ivan G, Szigeti-Csucs N, Olah M, Nagy GM, Goth MI. Treatment of pituitary tumors: dopamine agonists. Endocrine. 2005; 28(1):101-110.
- Ibáñez-Costa A., et al. BIM-23A760 influences key functional endpoints in pituitary adenomas and normal pituitaries: molecular mechanisms underlying the differential response in adenomas. Sci Rep. 2017 Feb 9;7:42002. doi: 10.1038/srep42002.
- Saveanu A, Jaquet P, Brue T, Barlier A. Relevance of coexpression of somatostatin and dopamine D2 receptors in pituitary adenomas. Mol Cell Endocrinol. 2008 May 14;286(1-2):206-13. doi:10.1016/j.mce.2007.12.008. Epub 2007 Dec 23.
- Culler MD. Somatostatin-dopamine chimeras: a novel approach to treatment of neuroendocrine tumors. Horm. Metab. Res. 2011 Nov;43(12):854-857.
- Florio T, Barbieri F, Spaziante R, et al. Efficacy of a dopamine-somatostatin chimeric molecule, BIM-23A760, in the control of cell growth from primary cultures of human non-functioning pituitary adenomas: a multi-center study. Endocr. Relat. Cancer. 2008 Jun;15(2):583-596.
- Greenman Y. Management of endocrine disease: Present and future perspectives for medical therapy of nonfunctioning pituitary adenomas. Eur. J. Endocrinol. 2017 Sep;177(3):R113-R124.
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Heather Halem serves as Vice President, Research at Tiburio Therapeutics. She brings more than 17 years of pharmaceutical experience to Tiburio with strong expertise in rare endocrine and metabolic diseases, oncology, and in developing pharmacology models of disease. A senior, cross-functional project team leader, she has direct experience leading research programs that have taken molecules from early identification to clinic candidates. Prior to joining Tiburio, she spent 15 years at Ipsen Bioscience, serving most recently as Director, Endocrine Modeling. In the role, she led research project teams from early stage target identification and concept assessment through lead optimization and into the clinic. She also initiated and directed in vivo biology research efforts and strategies for all endocrine projects. Previously, she was a senior scientist at Genome Therapeutics where she identified novel gene targets for neurodegenerative diseases and she also served as a postdoctoral research fellow at Massachusetts General Hospital. Dr. Halem earned her BA in Biology from Macalester College in St. Paul, MN, and her PhD in Biology from Boston University. She holds two patents and has authored numerous papers in leading peer-reviewed journals.

Dr. Michael D. Culler is the Scientific Founder and Chairman of the Scientific Advisory Board at Tiburio Therapeutics. He brings over 35 years of research experience and is an internationally recognized expert in endocrinology,metabolic disorders, and peptide therapeutics with over 200 publications and 22 patents. He was formerly Vice President of Endocrinology Research for Ipsen, where he led programs in diverse endocrine/ metabolic areas that resulted in five compounds reaching clinical development. He is a Co-founder of Rhythm Pharmaceuticals, and he led the preclinical programs that produced the key assets for both Rhythm (relamorelin and setmelanotide) and Radius Pharma (abaloparatide; Tymlos). Prior to joining industry, he conducted basic research in neuroendocrine physiology as a staff scientist at the National Institutes of Health.
Total Page Views: 4550










