Issue:June 2013
CELL CULTURE MEDIA - Addressing Variability in Dry Powder Mammalian Cell Culture Media
Introduction
Mammalian cell culture technology has become a major field on its own within the biopharmaceutical industry as the quality of culture media has a great impact on many important processes, ranging from cell growth in the R&D setting to critical quality attributes of new biological entities (NBEs).1,2 Biotherapeutics, such as monoclonal antibodies and recombinant proteins, are being produced in manufacturing facilities that utilize largescale cell culture production processes for both clinical and commercial use, and the bioprocessing industry is increasingly relying on ready-to-use dry powder media.1
The ability to deliver high-quality, reproducible good manufacturing practice (GMP) cell culture media relies on three critical elements: formulation optimization, quality of raw materials, and the production process. Although two batches of media may technically contain the same formulation, cell culture performance may be vastly different if the quality of the raw materials and/or the production process differs. The proper selection, qualification, and pre-processing of raw materials is essential for ensuring that the starting materials are of the highest quality in support of optimal media performance; the production process, including milling, mixing, and packaging, must be controlled and gentle enough to avoid degradation of the raw materials. It is therefore critical for media manufacturers to have a process in place that can consistently deliver raw material and process quality in order to be able to deliver batch-to-batch consistent dry powder cell culture media that are scalable from kilograms to tons.
Dulbecco’s Modified Eagle Medium (DMEM/F12) is a commonly used mammalian cell culture medium that contains a standard dry powder formulation among suppliers. Merck Millipore conducted a DMEM/F12 benchmarking study in order to assess the variability of this common medium among suppliers despite having a standard formulation. Dry powder DMEM/F12 was obtained from several suppliers, and numerous physicochemical and performance tests were conducted.
Not So Standard
Despite having a standard formulation, DMEM/F12 dry powder media produced by various suppliers appear very different as the powder ranges in color from white to pale yellow to pink. Because the formulation is the same, these color differences are likely the result of variations in the quality of raw materials and/or production processes used by the various suppliers, and imply that important physico-chemical properties, including pH, osmolality, humidity, and particle size distribution, may also vary across samples. Each of these properties is a critical parameter in mammalian cell culture as even small deviations could impact media performance.
Maintaining optimal pH (7.4) and osmolality (0.29 osmol/kg) of culture media is critical for cell viability and growth. One cell culture medium, Supplier S, varied significantly in both of these parameters; in particular, the osmolality of this sample was, on average, 0.4 below the standard osmolality expected based on the DMEM/F12 recipe. No precipitates were observed in any of the samples following solubilization; therefore, all powders were very soluble.
The water content, or humidity, in dry powder media has a direct impact on stability and storage. In addition to selecting the right packaging materials, it is important for media manufacturers to have an optimized production process in place, one that begins with and maintains a very low water content (~1%) to avoid supporting microbiological growth throughout production and storage. For this reason, water content should be routinely monitored throughout production. All DMEM/F12 samples were analyzed for water titer by Karl Fischer titration. Variability for humidity was observed among the samples, with a few approaching or exceeding a water titer of 1.2%.
Particle size distribution is a vital element that must be controlled in the production process because it has implications for demixing effects, solubility, oxidation, and microbial degradation as dry powders are not produced under sterile conditions. Each DMEM/F12 media sample was analyzed for particle size distribution using air jet sieving, and two types of particle size reduction effects were observed: one group of samples followed a linear distribution; another group of samples more closely followed a logarithmic scale. Demixing effects are observed to be lower for dry powder cell culture media that follows a more linear distribution.
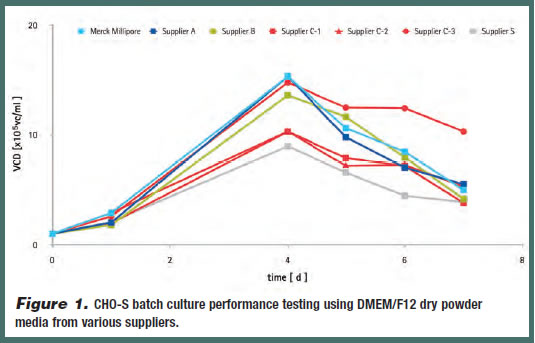
In addition to the pysico-chemical characterization assays, Chinese Hamster Ovary (CHO-S) batch culture performance testing was conducted to measure media performance. Approximately 1×105 viable CHO-S cells per mL were seeded and allowed to grow over several days. Viable cell density varied by as much as 50% across DMEM/F12 samples after four days (Figure 1).
Together, the physico-chemical and performance data from this DMEM/F12 benchmarking study conclude that “standard” formulation dry powder cell culture media are not in fact standard in their physicochemical and performance characteristics across suppliers. In addition to formulation optimization, media manufacturers must also have the right high-quality raw materials and production processes in place for optimal media performance.
Predicting Media Performance
Although informative for measuring media quality and performance, the analytical assessments used in the DMEM/F12 benchmarking study are very labor-intensive and time-consuming, especially if full testing is run for each new batch of medium. Merck Millipore has adopted much simpler “fingerprinting” technology that can predict media performance quickly and accurately without requiring full analytical testing. These methods include principle component analysis (PCA), nuclear magnetic resonance (NMR) profiling, and ultra performance liquid chromatography (UPLC).
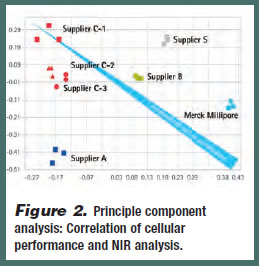
Principle component analysis is a standard tool used in data analysis that extracts relevant information from complex data sets. With respect to media performance, PCA revealed that nearinfrared spectra (NIR) of media samples contain enough physico-chemical information to be a robust predictor of media performance (Figure 2). This predictive tool can be used to identify quality differences and lot-to-lot variability among batches. A particular PCA score does not necessarily define high- or lowperforming media; rather, experience is needed from historical batches so that a particular PCA score can be mapped to higher and lower performing batches. In this model, an increase in cell culture performance is predicted as data moves from the upper left hand quadrant to the lower right hand quadrant.
If PCA testing predicts low performance, then the issue with the media could lie with impurities in the raw materials or with degradation of the raw materials as a result of the production process. These issues can be further analyzed using methods such as NMR profiling and UPLC – and then appropriately addressed.
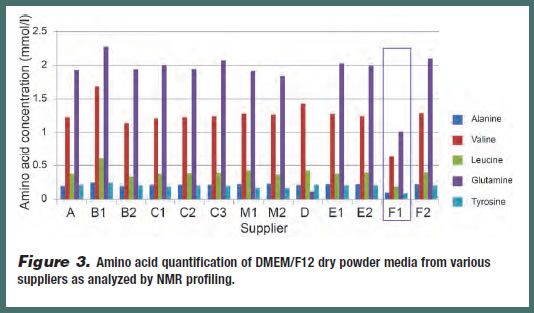
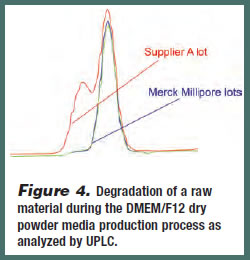
NMR profiling was conducted on an extended set of 13 different batches of DMEM/F12 media from various suppliers to quantify the amount of each amino acid present in each sample. Of this set, five amino acids – L-alanine, L-valine, Lleucine, L-glutamine and L-tyrosine – proved to be of particular interest due the variability of their concentration among some of the samples (Figure 3). For example, some batches of media were found to contain only half the concentration of L-valine and L-glutamine as predicted by the DMEM/F12 formulation. UPLC was conducted on the same set of DMEM/F12 media to identify potential impurities that could impact performance (Figure 4). A pre-peak was identified in one of the media batches, which suggests that degradation of a raw material during the production process had occurred (this was later confirmed by NMR analysis). Therefore, once a lowperforming batch of a medium has been identified, or predicted by PCA testing, potential impurities can be qualitatively and quantitatively analyzed.
In summary, high-quality raw materials, a gentle production process that preserves these critical raw materials from degradation, and having the right analytics in place for predicting media quality are all highly desired attributes for media manufacturers. These attributes are critical for the ability of manufacturers to deliver dry powder cell culture media that are consistent from batch-to-batch and scalable from kilograms to tons.
From Kilograms to Tons
Scaling up the production of dry powder cell culture media is an important topic for most large-scale biopharmaceutical producers because of the need to ensure batch-to-batch consistency of these large-scale productions. Merck Millipore frequently supplies tailored cell culture media in batches of up to 2.5 tons using state-of-the-art cGMP production facilities and raw materials sourced from qualified suppliers who can fulfill the requirements for our quality standards. These raw material qualification standards ensure batch-to-batch consistent delivery of the individual ingredients.
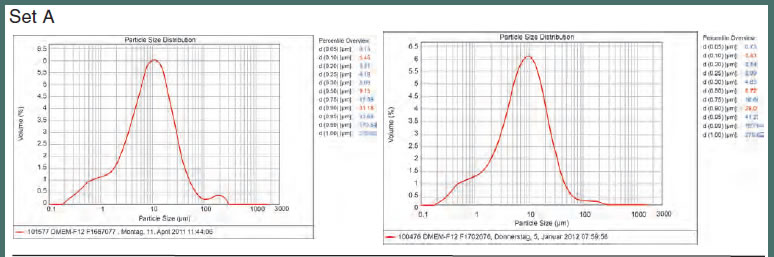
Merck Millipore assessed the reproducibility and scalability of DMEM/F12 dry powder medium, ranging from 1 kg to up to 2.5 tons, using only qualified raw materials. Dry powder medium was produced from one set of raw materials (set A) with pin-mill technology as lab-scale (up to 1 kg), mid-scale (up to 100 kg), and large-scale (up to 2.5 tons) batches. Using the same formulation and production process, but a completely different set of raw materials (raw material set B, purchased 6 months later) another large-scale batch was produced within 6 months. Each batch was then evaluated for pH value, osmolality, solubility, particle size distribution, and cellular performance.
All of the physico-chemical parameters were similar, regardless of the scale or raw material sets used. As shown in Figure 5, particle size consistency remained nearly identical from pilot material to large-scale production or when different batches of raw materials were used (raw material sets A and B), demonstrating both scalability and batchto- batch consistency, respectively. These data confirm that the scale-up and largescale production of dry powder cell culture media at Merck Millipore is a very highly controlled process. Additionally, a worldclass battery of analytics is used to achieve high levels of reliability and security.
Conclusion
The DMEM/F12 benchmarking study demonstrated that despite having a standard formulation, significant variability exists among dry powder media produced from different suppliers. Thus, formulation alone does not determine media performance; the quality of raw materials and the production process play critical roles in the delivery of consistent, high-quality GMP dry powder cell culture media. These elements are also critical for ensuring scalability while preserving the desired parameters.
There are three factors that contribute to the success of quality raw materials: a raw material manufacturing process that adheres to best practices; a raw material qualification program that ensures consistency of the individual components, and a raw material pre-treatment process that ensures each component has the proper surface area for optimal solubility. Batchto- batch consistency begins with consistency of the raw materials used. Additionally, there are three factors that contribute to a successful production process: a gentle milling technology that does not degrade the raw materials, an optimal mixing time, and a packaging process that controls for humidity. Merck Millipore has a very tightly controlled process in place that incorporates all of these elements, enabling smooth transitions from pilot-scale to commercial-scale cell culture production.
References
1. Butler M. Animal cell cultures: recent achievements and perspectives in the production of biopharmaceuticals. Appl Microbiol Biotechnol. 2005;68:283- 291.
2. Li F, et al. Cell culture processes for monoclonal antibody production. MAbs. 2010;2(5):466-479.

Jörg von Hagen, PhD
Head of R&D
Process Development
Merck Millipore
Dr. Jörg von Hagen is Head of Merck Millipore Process Development R&D in Darmstadt (Germany). Having studied biotechnology and signal transduction in Giessen and Darmstadt, he earned his academic degree with an award-winning thesis in 2001. Dr. von Hagen has more than 20 years of practical expertise in biotechnology, especially in molecular cell biology and proteomics.
Total Page Views: 6990