Issue:June 2019
BIOSIMILAR DEVELOPMENT - Approval of Biosimilar Medicines Through Totality of the Evidence
INTRODUCTION
A biosimilar medicine is designed to match the structure, function, and clinical effect of an already licensed biologic medicine – commonly referred to as a “reference” medicine. The US FDA, EMA, and WHO all apply the same general definition for a biosimilar: a biologic medicine that matches a reference biologic that has been previously approved by regulatory authorities.1-3 Biosimilar medicines are held to the same safety, purity, and potency standards as all biologic medicines.1,3-5 To establish biosimilarity, extensive analytical testing confirms structural and functional matching, while confirmatory clinical trials are conducted to show that the biosimilar matches its reference biologic in terms of safety and efficacy.4-6 As of May 2019, 19 biosimilar medicines have been approved by the FDA, and more than 60 have been approved by the EMA.7,8
Biosimilar medicines are developed and tested under the paradigm of the “totality of the evidence” criteria, which verifies the high similarity of biosimilar and reference medicines through head-to-head comparisons of multiple structural and functional parameters, as well as human pharmacokinetic (PK) and pharmacodynamics (PD) studies. This is then confirmed by limited but very directed clinical confirmation of efficacy and safety.5,6 This article outlines the evolution of biosimilar approvals and describes the process of analytical and clinical testing followed in the development of a biosimilar, with use of data from the scientific literature.
Biologics have been the fastest-growing class of pharmaceuticals in the US, offering treatments for many conditions that long eluded medicinal treatment.9 Many of the revenue-leading biologics (eg, Humira®/AbbVie, Remicade®/Janssen) are coming off patent. This is potentially paving the way for use of biosimilar medicines in several therapeutic classes, such as oncology, immunology, and endocrinology.10,11 With continued expansion of the market, experts forecast that biosimilar medicines could save $54 billion in spending on biologics over 10 years.12
Sandoz developed the first approved biosimilar in the world: human growth hormone, Omnitrope® (somatropin), approved in Europe in 2006.1,7 To gain approval, Sandoz presented data from analytical, preclinical, and clinical studies to the EMA confirming that the somatropin biosimilar matched the reference biologic (Genotropin®/Pfizer).10 Omnitrope was approved by the FDA in 2006 as a new drug that relied in part on reference product data (called the 505(b)(2) pathway) rather than as a biosimilar, because the legal basis for approving biosimilar medicines in the US did not exist at that time.10
DEVELOPING THE PATHWAY FOR BIOSIMILAR APPROVAL
Biosimilar medicines are designed to match the structure, function, and clinical performance of their reference biologic. Biosimilar development is possible because technology has advanced to the point that a new biological medicine can be designed very precisely. Moreover, analytical methods are much more sensitive and specific than a few decades ago, which led to a revolution in our ability to analyze protein structures.5
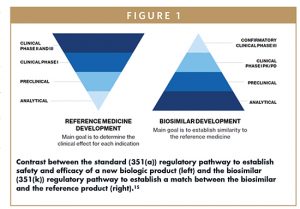
Unlike approval of new medicines, where the aim of the development program is to establish the safety and efficacy of a new molecular entity for treatment of a specific condition in a specific patient population, the goal of biosimilar development is to demonstrate, by means of head-to-head comparisons, that the biosimilar medicine matches the reference biologic very closely and that any differences that might exist have no impact on safety or efficacy.5 Experts realized that instead of mandating a single approach for all biological medicines regardless of structure or indication, it would be more appropriate to take a case-by-case approach for individual products including the implementation of study designs with optimally sensitive patient populations and endpoints.6,9,13
Multiple different types of comparative data are collected, including analytical tests, biological assays, human PK and PD studies (to assess what happens to the medicine in the body, or what happens to the body when exposed to the medicine, respectively), immunogenicity studies, and if necessary, limited human safety and efficacy studies. It is not necessary to repeat large human efficacy and safety studies because the safety and efficacy of a biosimilar is based on data established in studies of the reference biologic.5,6 This more specific and streamlined process became the “totality of the evidence” standard.4,5
The licensure process for biosimilar medicines in the US was established in 2010, when the Biologics Price Competition and Innovation Act (BPCIA) was enacted.14 The BPCIA was intended to enable approval of biosimilar medicines to increase treatment options, potentially save lives and decrease healthcare costs through market competition.3,14 The biosimilar pathway also intended to curtail unnecessary and potentially unethical testing in animals and humans.9,13 The quantity of data provided with a biosimilar application is very large, although more emphasis is placed on the analytical and biological function data than on clinical data (the opposite is true for reference biologic applications). The rigor of the FDA review is just as high for a biosimilar as was applied to the reference medicine when it was initially approved. Figure 1 illustrates how the biosimilar program differs from that for originator medicinal biologics.
In 2015, Zarxio® (filgrastimsndz/Sandoz) became the first approved biosimilar in the US, based on evidence from analytical, human PK, human PD, and confirmatory human safety and efficacy studies demonstrating biosimilarity to the reference biologic, Neupogen® (filgrastim/Amgen).5,8 Zarxio approval was followed by more than a dozen other biosimilars in the US, including, ErelziTM (etanercept-szzs/Sandoz) and HyrimozTM (adalimumab-adaz/Sandoz).8 Biosimilarity for Zarxio was based on 22 analytical methods evaluating 19 different attributes; more complex biosimilar medicines (eg, Erelzi) have required more than 50 analytical methods for more than 80 attributes.5
APPROVAL OF A BIOSIMILAR: “TOTALITY OF THE EVIDENCE”
Approval of a biosimilar is based on the “totality of the evidence” standard, which can be defined as the sum of data from analytical, preclinical, and clinical studies.5,6 According to the FDA:
“There is no one size fits all approach to biosimilar product development. The goal of a biosimilar development program is to use a “totality of the evidence” approach to demonstrate biosimilarity to the reference product, not to independently establish safety and effectiveness of the proposed biosimilar.”3
The FDA evaluates biosimilar medicines on a case-by-case basis, mandating or waiving certain types of studies based on the nature of the molecule and its intended use.3,5,6 The goal is to prove high similarity to the reference biologic, not to directly test the safety and efficacy of the biosimilar medicine.3,5,6
The totality of the evidence standard is accepted worldwide, including by the FDA, EMA, and WHO.4,5 Totality of the evidence signifies verification that patients can expect the same clinical performance when using the biosimilar as when using the reference biologic, and that there will be no clinically meaningful differences with respect to safety or effectiveness.5 In short, according to the FDA:
“…the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components … there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product.”6
DEVELOPING A BIOSIMILAR
Biologics, both reference and biosimilar medicines, are manufactured from living organisms. As such, it is normal to have lot-to-lot variability for all biological medicines. No lot of a biological medicine is an exact replica of prior lots, but all are within ranges that are known to be safe and efficacious.4,5 These ranges are the development target for the corresponding biosimilars. Likewise, a biosimilar is not a precise replica of its reference biologic, but the differences are acceptable if they are not clinically meaningful.1,3-6
Analytical Comparisons
The goal of the analytical evaluation is to ensure that the biosimilar is within variability of the reference biologic across the multiple assays used, and that any minor difference is not clinically relevant.
Analytical studies are intended to demonstrate a molecular (physicochemical) and functional (bioassay) match to a previously-approved reference biologic.4,5,16 Structural and functional attributes include primary structure (ie, identical primary amino acid sequence), higher order structure (the three-dimensional shape), biological activity (as measured by bioassays that can include receptor binding or cell-based assays), protein content, sub-visible particles, impurities, thermal stability, post-translational modifications including glycosylation and higher molecular-weight variants or aggregates.4,5,10
Any residual uncertainty from the analytical similarity assessment is examined for potential clinical relevance by means of human PK and PD studies, and if appropriate, in safety and efficacy studies.5,16 Analytical and functional data establishing molecular similarity are the foundation for the totality of the evidence model for biosimilar medicines.5
State-of-the-art analytical methods are used to characterize both the biosimilar and the reference biologic.5,10 Many analytical techniques in use today did not exist at the time of approval of the reference biologics. Additionally, these new analytical methods may bring to light batch-to-batch variability in reference biologics that was previously unrecognized.5 These analytical methods are more sensitive than clinical studies and enable biosimilar developers to detect sub-clinical differences, giving them increased importance in evaluating biosimilarity.6
Preclinical & Clinical Studies
Biosimilar medicines are tested in a stepwise fashion, with a targeted preclinical and/or clinical program following structural and functional characterization. The extent of the preclinical and/or clinical program depends on the residual uncertainty that may exist after the analytical and functional comparisons of the biosimilar and reference biologic.3-5,10,16 The targeted clinical development program is scientifically justified because the reference biologic has already been demonstrated to be safe and effective in the approved indications.2,4,5 The goal is not to prove it again with a biosimilar, but to confirm the absence of any clinically meaningful differences versus the reference biologic.4-6,10
In the clinical stage of biosimilar development, human PK, and, if applicable, PD studies are central. They are sensitive tools to ascertaining whether there are potential clinically relevant differences between a biosimilar and its reference biologic.5,13,16 Human PK studies are typically conducted in a healthy subject population because they are not receiving any other medications or have comorbid conditions that could confound the results.4,5 Immunogenicity studies are also conducted in a sensitive population and must be long enough in duration to allow development of antibodies after extended exposure to the biologic medicinal product. In addition, the studies must assess for the development of neutralizing antibodies that could have clinical implications.5
Confirmatory safety and efficacy studies may be conducted in a patient population, although again, these are designed to detect any clinically relevant differences that may exist, and not to reestablish safety or efficacy of the active substance.4,5 As a result, the studies use a sensitive sub-population and endpoint which may be different from those used to establish de novo the efficacy and safety of an active substance.6
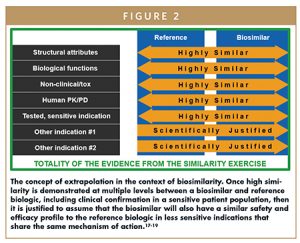
Extrapolation
In line with the aforementioned, it is not necessary to repeat studies in all indications for which the reference biologic is approved because the concept of extrapolation can be applied. Extrapolation allows approval of a biosimilar for other indications for which the reference biologic is approved, even if the biosimilar was not studied specifically on those indications.3,6,10 Extrapolation is not a mere assumption of efficacy and safety for a different indication; it must be rigorously supported by scientific evidence.3,6,10 The essence of extrapolation is that if high similarity is demonstrated in structural attributes, biological functions, human PK, human PD, and clinical efficacy and safety in a sensitive patient population (including immunogenicity), then it is justified to assume that the biosimilar will also have a similar safety and efficacy profile to the reference biologic in less sensitive indications that share the same mechanism of action.
The concept of extrapolation has been used by health authorities for decades. For example, when a major manufacturing change is introduced for an originator biologic that may result in changes in product characteristics. If the results confirm that the clinical performance has not changed, health authorities extrapolate and approve that biologic made with the process change for use with all indications, including those indications and populations that were not studied with post-change material. Clinical trials comparing the biosimilar to the reference biologic for each indication are not only unnecessary, but would also obviate the goals of expedited biosimilar approval (ie, the BPCIA).5,6 Further, extrapolation in the biosimilars class has become widely accepted by health authorities – continuing to play a substantial role in biosimilar approvals.6,17
Substitution & Interchangeability
Prescribers have the ability to prescribe the medication they believe is most appropriate for their patients. As such, they have the ability to switch from a reference biologic to a biosimilar at any point in time they deem appropriate. However, for a pharmacist to substitute a biosimilar for a reference biologic without first obtaining permission from the prescriber, the biosimilar must be designated by the FDA as “interchangeable.”
Interchangeable biologics are evaluated to the same level of quality standards as described previously.3,9 The FDA, however, has indicated that for products to be designated as interchangeable, additional, and different data will be required. In particular, for products administered to a patient more than once, manufacturers will need to conduct an additional clinical study in which patients are switched back and forth at least three times between the reference biologic and biosimilar. The concept of interchangeability is unique to the US – no other country has a separate interchangeability category. Many authorities believe that whether an approved biosimilar is designated interchangeable or not, the risk posed by switching between the biosimilar and the reference biologic is no greater than the risk posed by using the reference medicine only. To date, no company has sought an interchangeable product designation in the US for a biosimilar.20
Public Acceptance of Biosimilar Medicines
The mission of the FDA is to be “responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices.”21 Biosimilar development is strictly regulated by the FDA and each application undergoes very thorough review by the Agency based on different but similarly stringent requirements compared to their reference biologic. This ensures their quality, safety, and efficacy so patients and prescribers can expect the same benefits as the reference biologic. While the development and regulatory review of a reference biologic primarily focuses on the Phase III clinical data, the biosimilar development and review focus on comparisons to the reference biologic that are most sensitive to detect differences.3,5
Providers and patients must be educated about the biosimilar approval process to help them understand and then accept these products.1,3,5 This may be a challenge for prescribers as they are trained to understand and accept medicines based on extensive clinical data, such as directly observed improvement in a disease state. For establishing biosimilarity, the most important data are analytical test results comparing the structure and function of a biosimilar and reference biologic. Patient education is also important as biosimilar medicines are utilized to reduce costs and increase access. It must be carefully explained to patients that there will be no difference in efficacy or safety if they receive a biosimilar in place of a reference biologic.1,3,5,6
SUMMARY
Unlike new medicines that are approved on the basis of extensive clinical data, often in multiple indications and in different patient populations, biosimilar medicines are developed using the concept of totality of the evidence. This means biosimilar medicines and their reference biologics are compared analytically, functionally, and then in a more limited human clinical program to verify the high similarity of the two medicines.
Because this is a relatively new concept for providers and their patients, education is necessary to increase understanding of what these products are, how they are approved, and the fact that patients can expect matching safety and efficacy of a FDA approved biosimilar and the respective reference biologic. Knowledge and acceptance of biosimilar medicines are critical to helping fulfill the promise of the BPCIA for improving access to high-quality medicines while decreasing societal healthcare costs.
REFERENCES
- European Medicines Agency (EMA). Biosimilar medicines. Available at: https://www.ema.europa.eu/humanregulatory/overview/biosimilar-medicines. Accessed November 7, 2018.
- World_Health Organization (WHO). Biologicals: similar biotherapeutic products (2017). Available at: http://www.who.int/biologicals/biotherapeutics/similar_biotherapeutic_products/en. Accessed December 9, 2018.
- US Food & Drug Administration (FDA). Biosimilar development, review, and approval (2017). Available at: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/ucm580429.htm. Accessed December 6, 2018.
- Markus, R., et al. Developing the Totality of Evidence for Biosimilars: Regulatory Considerations and Building Confidence for the Healthcare Community. BioDrugs 31, 175-187 (2017).
- Cohen, H et al. Totality of evidence and the role of clinical studies in establishing biosimilarity. in Biosimilars. AAPS Advances in the Pharmaceutical Sciences Series, Vol. 34 (2018).
- US Department of Health and Human Services, F.a.D.A.F. Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry (2015). Available at: https://www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Accessed December 8, 2018.
- Rothwell Figg. Biologics & Biosimilars. FDA’s Biosimilar Approvals Accelerate in 2018: How the U.S. Compares to Europe on Biosimilar Approvals and Products In the Pipeline. Available at: https://www.biosimilarsip.com/2019/05/07/how-the-u-s-compares-to-europe-on-biosimilar-approvals-and-products-in-the-pipeline-4/. Accessed May 7, 2019.
- US Food & Drug Administration (FDA). Biosimilar product information (2019). Available at: https://www.fda.gov/drugs/biosimilars/biosimilar-product-information. Accessed May 7, 2019.
- Kozlowski, S., Woodcock, J., Midthun, K. & Sherman, R.B. Developing the nation’s biosimilars program. N Engl J Med 365, 385-388 (2011).
- Schiestl, M., et al. Ten years of biosimilars in Europe: development and evolution of the regulatory pathways. Drug Des Devel Ther 11, 1509-1515 (2017).
- Tariman, J.D. Biosimilars: Exploring the History, Science, and Progress. Clin J Oncol Nurs 22, 5-12 (2018).
- Walker, N. Pharmaceutical Manufacturing. The rise of biosimilars (2016). Available at: https://www.pharmamanufacturing.com/articles/2016/the-rise-ofbiosimilars. Accessed December 9, 2018.
- Warren, J.B. Generics, chemisimilars and biosimilars: is clinical testing fit for purpose? Br J Clin Pharmacol 75, 7-14 (2013).
- US Food & Drug Administration (FDA). Biosimilars: biosimilars are safe, effective treatment options (2018). Available at: https://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/ approvalapplications/therapeuticbiologicapplications/biosimilars/default.htm. Accessed December 6, 2018.
- Christl, L. et al. Overview of the regulatory framework and FDA’s guidance for the development and approval of biosimilar and interchangeable products in the US. https://www.fda.gov/downloads/AboutFDA/WorkingatFDA/FellowshipInternshipGraduateFaculty Programs/PharmacyStudentExperientialProgramCDER/UCM587522.pdf. Accessed December 16, 2018.
- US Food and Drug Administration (FDA). Biosimilar development process [poster]. Available at: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDeveloped andApproved/ApprovalApplications/TherapeuticBiologcApplications/Biosimilars/UCM607696.pdf. Accessed December 6, 2018.
- European Medicines Agency (EMA). Guideline on similar biological medicinal products containing monoclonal antibodies – non-clinical and clinical issues (2012). Available at: https://www.ema.europa.eu/documents/scientificguideline/guidelinesimilar-biological-medicinal-productscontaining-monoclonal-antibodiesnon-clinical_en.pdf. Accessed January 30, 2019.
- Kurki, P., Bielsky, M.C. & Working Party on Similar Biological Medicinal Products of Committee for Medicinal Products for Human, U. ECCO position challenged by European drug regulators. J Crohns Colitis 8, 258 (2014).
- Weise, M. Biosimilars: what clinicians should know. Blood 120, 5111-5117 (2012).
- Cohen, H.P. Switching Reference Medicines to Biosimilars: A Systematic Literature Review of Clinical Outcomes. Drugs 78, 463-478 (2018).
- US Food & Drug Administration (FDA). What We Do. Available at: https://www.fda.gov/aboutfda/whatwedo/. Accessed January 2, 2019.
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Hillel P. Cohen is Executive Director of Scientific Affairs at Sandoz, helping explain the principles of biosimilars and biosimilar policies to the healthcare community, patient advocacy groups, and health authorities. Dr. Cohen led Sandoz efforts for the first biosimilar presentation (Zarxio®) to an FDA advisory committee and participated in BsUFA 2 negotiations on behalf of industry. He helped co-found the Biosimilars Forum, where he is currently co-chair of the education committee. He is also a member of the education and regulatory committees of the Biosimilars Council, a division of the Association for Affordable Medicines. Dr. Cohen earned his BA from New York University and his PhD in Biology from Dartmouth.
Total Page Views: 18939