Issue:September 2013
BIOAVAILABILITY ENHANCEMENT - Navigating a Broad Spectrum of Solubilization Technologies: Part I of III
In the past two columns, The Second Quadrant focused on excipients and the roles they play in the quest to overcome bioavailability challenges of poorly soluble drugs. For the next three issues, we will hear perspectives of experts whose respective companies provide solubility-enhancement technologies to the industry. Various old and new technologies exist, most of which are represented by the participants in this column. The goal of this particular series is to provide information to assist in selecting appropriate solubilization technologies for different compounds.
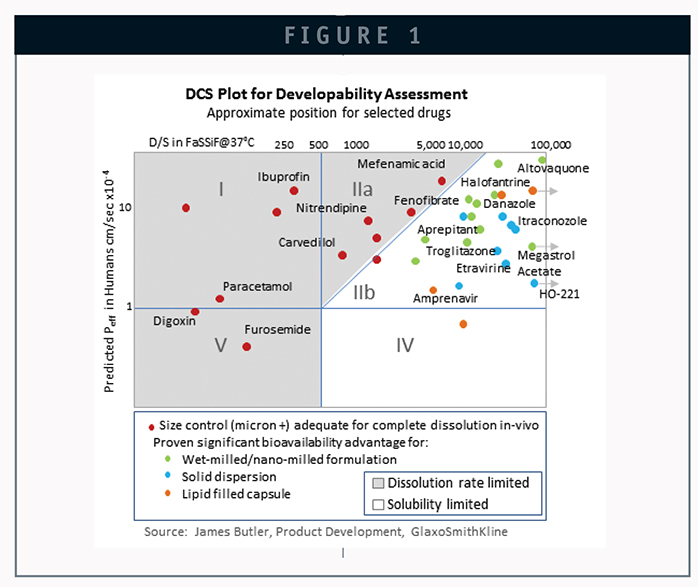
For those with limited experience in the delivery of poorly soluble compounds, the selection of the ideal technology for a particular drug candidate can be daunting. Since the 1970s, many different approaches have been attempted and developed, and numerous reviews have been published. The BCS (Biopharmaceutical Classification System) was developed as a regulatory tool to give guidelines and recommend classification of drugs into four quadrants based on dosage form dissolution and the solubility and permeability characteristics of the drug substance. The addition of DCS (Developmental Classification System) further partitioned class II compounds based on dissolution rate and solubility, with the goal of assisting in the selection of appropriate delivery approaches for new drug candidates. An analysis by James Butler of GlaxoSmithKline features selected drugs and the respective solubilization technologies used in the four main quadrants of the DCS system (Figure 1).1 This figure provides interesting insight into where the different technologies have historically been applied to achieve improved bioavailability. From this map, it can be seen that while particle size reduction has dominated for dissolution rate-limited drugs, a broader range of technology options is represented when addressing solubility-limited drugs. In particular, it is clear that a number of approaches have been successfully employed to deliver DCS class IIb compounds. So, the question still exists as to what technology should be selected for a new compound with poor bioavailability in its crystalline form.
The goal of this 3-part series of The Second Quadrant is to add to our collective understanding of which specific solubilization technologies have achieved the best results in the past and which offer the greatest promise today and going forward. To help readers better navigate the available options, representatives of a broad range of technologies provide their views on a number of questions important to those considering different approaches for a poorly soluble compound. The companies represented include those offering lipid-based solubilization, amorphous solid dispersions, particle size reduction, particle engineering, nanoparticles, and co-crystallization. I am grateful for the efforts of each of the contributors and their thoughtful responses.
SELECTION CRITERIA
Q: What API characteristics or dosage form requirements can be used to select the right solubilization technology?
 Dan Dobry: When choosing a formulation approach for low-solubility compounds, it’s important to understand the following properties: 1) tendency of the molecule to crystallize, 2) lipophilicity, 3) dose, 4) solubility (aqueous and organic), and 5) presence of ionizable groups. Based on our experience of formulating over a thousand compounds, Bend Research has developed a number of flow charts and other guidances for choosing a formulation using a technology agnostic approach. Oral dosage form selection is generally dependent on the phase of development. Many of our clients prefer suspensions or capsules for Phase I because these forms make for facile dose escalation. Later stages of development can take the form of tablets, capsules, or modified-release dosage forms. The choice is dependent on many factors, including pharmacokinetic performance and target product profile or image.We work closely with our partners and clients, using science and engineering fundamentals to provide reliable, rapid, low-risk compound advancement.
Dan Dobry: When choosing a formulation approach for low-solubility compounds, it’s important to understand the following properties: 1) tendency of the molecule to crystallize, 2) lipophilicity, 3) dose, 4) solubility (aqueous and organic), and 5) presence of ionizable groups. Based on our experience of formulating over a thousand compounds, Bend Research has developed a number of flow charts and other guidances for choosing a formulation using a technology agnostic approach. Oral dosage form selection is generally dependent on the phase of development. Many of our clients prefer suspensions or capsules for Phase I because these forms make for facile dose escalation. Later stages of development can take the form of tablets, capsules, or modified-release dosage forms. The choice is dependent on many factors, including pharmacokinetic performance and target product profile or image.We work closely with our partners and clients, using science and engineering fundamentals to provide reliable, rapid, low-risk compound advancement.
 Tom Dürig: Our major solubilization technologies are polymer based and involve the formation of amorphous drug polymer dispersions. Critical drug characteristics include dose, glass transition and melting temperatures, ionic behavior (drug pKa), stability, and hydrophobicity as indicated by partition coefficient (log P). Dose-per-unit dosage form is particularly critical as solid dispersion usually require a large portion of polymer (typically higher than 50%) for stabilization and solubilization. When the dose exceeds 400 mg, this can lead to obvious patient administration and compliance issues due to dosage form size. Additionally, the dose-to-solubility ratio is a key indicator of the solubility challenge that a given compound presents. We typically adjust polymer loading based on crystallization propensity, which can be approximated by the ratio of the glass transition and melting temperatures. If thermal processing, such as hot-melt extrusion (HME), is desired, then drug melting points below 180°C and good thermal stability are valuable.
Tom Dürig: Our major solubilization technologies are polymer based and involve the formation of amorphous drug polymer dispersions. Critical drug characteristics include dose, glass transition and melting temperatures, ionic behavior (drug pKa), stability, and hydrophobicity as indicated by partition coefficient (log P). Dose-per-unit dosage form is particularly critical as solid dispersion usually require a large portion of polymer (typically higher than 50%) for stabilization and solubilization. When the dose exceeds 400 mg, this can lead to obvious patient administration and compliance issues due to dosage form size. Additionally, the dose-to-solubility ratio is a key indicator of the solubility challenge that a given compound presents. We typically adjust polymer loading based on crystallization propensity, which can be approximated by the ratio of the glass transition and melting temperatures. If thermal processing, such as hot-melt extrusion (HME), is desired, then drug melting points below 180°C and good thermal stability are valuable.
For spray-drying, we focus on drug and polymer combinations with good solubility in organic solvents, such as acetone and methanol. Drug ionization behavior (pKa) is important when ionic polymers are used as solid-dispersion carriers because the ionic interaction between API and polymer could be the key factor that impacts dissolution and stability of the solid dispersion. For instance, we have achieved particularly good solubility results for weak base compounds, like itraconazole, using AquaSolveTM L HPMCAS that has more succinoyl groups. In contrast, for weak acids (neutral or basic pKa), such as felodipine and ezetimibe, AquaSolve H HPMCAS with more acetyl groups is particularly effective in improving solubiliy.
 Joan Feixas: Pharmaceutical cocrystals (multicomponent crystal API – coformer) are currently recognized as a preferred alternative to optimize API physicochemical properties, such as solubility and dissolution rate without chemical modification of the drug substance. This technology is especially indicated for nonionizable or poorly ionizable APIs. A cocrystal can also be used instead of a salt when it is not possible to obtain a solid or crystalline form of the salt, when the salt formation results in chemical degradation or when there is a risk of crystalline transformation to a less-soluble form during formulation or storage.
Joan Feixas: Pharmaceutical cocrystals (multicomponent crystal API – coformer) are currently recognized as a preferred alternative to optimize API physicochemical properties, such as solubility and dissolution rate without chemical modification of the drug substance. This technology is especially indicated for nonionizable or poorly ionizable APIs. A cocrystal can also be used instead of a salt when it is not possible to obtain a solid or crystalline form of the salt, when the salt formation results in chemical degradation or when there is a risk of crystalline transformation to a less-soluble form during formulation or storage.
 Filipe Gaspar: Physical and chemical properties of the drug substance, such as melting point; logP; chemical, thermal, and physical stabilities; release mode; and dose level, all play an important role when selecting the stabilization platform and the formulation approach. Soft gels may apply for low-dosage and very hydrophobic compounds, nanoparticles may apply when bioavailability is dictated by dissolution rate, and solid dispersions seem to have the broadest application of all. Once a platform is selected, the question is to decide on the manufacturing technology. For solid dispersions, spray-drying works for nearly all compounds and HME, when applicable, is likely to be a more cost effective technology.
Filipe Gaspar: Physical and chemical properties of the drug substance, such as melting point; logP; chemical, thermal, and physical stabilities; release mode; and dose level, all play an important role when selecting the stabilization platform and the formulation approach. Soft gels may apply for low-dosage and very hydrophobic compounds, nanoparticles may apply when bioavailability is dictated by dissolution rate, and solid dispersions seem to have the broadest application of all. Once a platform is selected, the question is to decide on the manufacturing technology. For solid dispersions, spray-drying works for nearly all compounds and HME, when applicable, is likely to be a more cost effective technology.
 Robert Hoerr: The ability to get a BCS class II or IV compound into a solubilizable form is what guides most decisions to use solubilization technologies. Nanocopoeia is a therapeutic particle engineering company providing nano-enabled particle design, services, and equipment to the pharmaceutical industry. Specific to ElectroNanosprayTM (ENS) nanoformulation, the most important API considerations are solubility in organic solvents, the need to reduce or eliminate problematic excipients, and the desire to protect, stabilize, and/or control the release of the API at the point of delivery.
Robert Hoerr: The ability to get a BCS class II or IV compound into a solubilizable form is what guides most decisions to use solubilization technologies. Nanocopoeia is a therapeutic particle engineering company providing nano-enabled particle design, services, and equipment to the pharmaceutical industry. Specific to ElectroNanosprayTM (ENS) nanoformulation, the most important API considerations are solubility in organic solvents, the need to reduce or eliminate problematic excipients, and the desire to protect, stabilize, and/or control the release of the API at the point of delivery.
 Keith Hutchison: The combination of a number of key physical, chemical, and biopharmaceutical API properties tends to drive formula development and optimization in lipid-based liquid/semisolid compositions for poorly soluble compounds. Key chemical and physical API properties include molecular weight, crystalline melting temperature, aqueous solubility, pKa, apparent partition coefficient, and polarity. Beyond these fundamental characteristics, compound solubility in lipid excipients, surfactants, and co-solvents is important to achieve adequate drug loading per unit dose. In some cases, API in suspension in a lipidbased vehicle can be used to deliver higher doses.
Keith Hutchison: The combination of a number of key physical, chemical, and biopharmaceutical API properties tends to drive formula development and optimization in lipid-based liquid/semisolid compositions for poorly soluble compounds. Key chemical and physical API properties include molecular weight, crystalline melting temperature, aqueous solubility, pKa, apparent partition coefficient, and polarity. Beyond these fundamental characteristics, compound solubility in lipid excipients, surfactants, and co-solvents is important to achieve adequate drug loading per unit dose. In some cases, API in suspension in a lipidbased vehicle can be used to deliver higher doses.
 Dave Miller: The most successful applications of DisperSol’s KinetiSol® technology are those involving insoluble compounds that require supersaturating delivery systems to enhance oral absorption and are also difficult to process by spray-drying and melt extrusion. Specifically, our technology is amenable to the production of amorphous solid dispersion systems with insoluble compounds having one or more of the following issues: high melting point, thermal sensitivity, poor organic solvent solubility, and/or requiring concentrationenhancing carrier polymer(s) that are thermally labile or highly viscous. KinetiSol is a novel fusion-based technology with very brief processing times at elevated temperatures (< 5 sec); therefore, the process is able to generate amorphous solid dispersions with thermally labile compounds and polymers that would degrade by melt extrusion. The process also offers mixing intensity an order of magnitude greater than melt extrusion, which enables compound solubilization in a carrier polymer well below its melting point and enables melt processing of highly viscous polymers without the need for plasticizers. The technology offers all the advantages of non-solvent processing and is particularly applicable when poor organic solvent solubility limits the use of spray- drying. When compounds exhibit high melting points or thermal sensitivity in addition
Dave Miller: The most successful applications of DisperSol’s KinetiSol® technology are those involving insoluble compounds that require supersaturating delivery systems to enhance oral absorption and are also difficult to process by spray-drying and melt extrusion. Specifically, our technology is amenable to the production of amorphous solid dispersion systems with insoluble compounds having one or more of the following issues: high melting point, thermal sensitivity, poor organic solvent solubility, and/or requiring concentrationenhancing carrier polymer(s) that are thermally labile or highly viscous. KinetiSol is a novel fusion-based technology with very brief processing times at elevated temperatures (< 5 sec); therefore, the process is able to generate amorphous solid dispersions with thermally labile compounds and polymers that would degrade by melt extrusion. The process also offers mixing intensity an order of magnitude greater than melt extrusion, which enables compound solubilization in a carrier polymer well below its melting point and enables melt processing of highly viscous polymers without the need for plasticizers. The technology offers all the advantages of non-solvent processing and is particularly applicable when poor organic solvent solubility limits the use of spray- drying. When compounds exhibit high melting points or thermal sensitivity in addition
to poor organic solvent solubility, KinetiSol if often the only viable amorphous solid dispersion processing technology.
 Peter Nelson: The decision to evaluate particle size reduction via micronization (jet-milling) and/or mechanical milling can typically be made based upon three major API characteristics/requirements: particle size of the synthesized API, crystallinity, and thermal stability. If the particle size of the synthesized API is among the solubility rate-limiting factors, micronization or mechanical milling canbe employed to effectively reduce the particle size to the desired range. This assumes, of course, that the required particle size is attainable via dry milling. If the API is required to be in a crystalline form to be effectively formulated, micronization is again a viable option. Our studies have shown that the micronization process retains drug substance crystallinity and that amorphous content (when present) is typically negligible. Finally, drug substances exhibiting poor thermal stability may benefit from the micronization process. Jet milling is a thermally neutral process.
Peter Nelson: The decision to evaluate particle size reduction via micronization (jet-milling) and/or mechanical milling can typically be made based upon three major API characteristics/requirements: particle size of the synthesized API, crystallinity, and thermal stability. If the particle size of the synthesized API is among the solubility rate-limiting factors, micronization or mechanical milling canbe employed to effectively reduce the particle size to the desired range. This assumes, of course, that the required particle size is attainable via dry milling. If the API is required to be in a crystalline form to be effectively formulated, micronization is again a viable option. Our studies have shown that the micronization process retains drug substance crystallinity and that amorphous content (when present) is typically negligible. Finally, drug substances exhibiting poor thermal stability may benefit from the micronization process. Jet milling is a thermally neutral process.
 Deepak Tiwari: At Particle Sciences, we have a number of solubilization approaches ranging from in silico design to solid solutions to lipid-based systems, such as LyoCells®. So, it is more of a question to which technology do the API’s characteristics drive one toward. For instance, a heat-stable highly potent compound with a positive log P naturally drives toward HME, while a heat-labile molecule with a positive log P can be converted to amorphous form utilizing spray drying. Another approach for a relatively labile molecule with good lipid solubility would warrant looking at LyoCells, and of course, a classic BCS II molecule should always be evaluated for its amenability to crystalline nanoparticles.
Deepak Tiwari: At Particle Sciences, we have a number of solubilization approaches ranging from in silico design to solid solutions to lipid-based systems, such as LyoCells®. So, it is more of a question to which technology do the API’s characteristics drive one toward. For instance, a heat-stable highly potent compound with a positive log P naturally drives toward HME, while a heat-labile molecule with a positive log P can be converted to amorphous form utilizing spray drying. Another approach for a relatively labile molecule with good lipid solubility would warrant looking at LyoCells, and of course, a classic BCS II molecule should always be evaluated for its amenability to crystalline nanoparticles.
INTRACTABLE APIS
Q: Are there physical & chemical properties that typify an “intractable” compound?
Dan Dobry: Our experience at Bend Research is that many of the factors that typify an intractable compound can be dealt with using a science-based approach. That being said, an intractable compound can be typified in several ways through the lens of performance, manufacturability, and stability. For example, compounds with extremely low aqueous solubility (< 1 μg/mL) or low permeability (typified by ionic state, molecular weight, and polar surface area), may be considered intractable from a performance perspective. However, the appropriate enabling technology can often achieve the desired oral absorption. Intractable compounds from a manufacturability perspective tend to be typified by low solubility in processing solvents, which is generally translatedto having extremely high melting points (> 230°C, generally considered to be brick dust) or compounds that have very low glass transition temperatures (< 50°C). Formulation and process approaches can be utilized to enable these compounds in many cases. From a physical and chemical stability perspective, compounds that have extremely low glass transition temperatures (< 50°C), high melting point to glass transition temperature ratios (> 1.35 K/K), and chemical functionality known to be labile to hydrolysis or oxidation tend to be considered intractable. In these cases, functional excipients will be required to stabilize either the amorphous state or to block the chemical reactivity and lead to a stable product. It is worth noting that despite a compound having intractable properties, dose can often be the leading factor in exacerbating or minimizing the extent of the challenge to deliver the molecule.
Tom Dürig: The most intractable properties would include high crystallinelattice energy and high lipophilicity. APIs that have extremely low solubility in all commonly used spray-drying solvents and/or that demonstrate thermal instability could present significant challenges during process development. Joan Feixas: Regarding the question of intractable compounds, almost all the APIs are possible candidates to be used to form cocrystals. However, the probability of success decreases with fewer chemical functions bearing hydrogen-bonding acceptors or donors, fewer aromatic rings to form pi-stacking, or the presence of a high structure flexibility. Filipe Gaspar: The most challenging compounds include compounds from BCS class 4 and those that combine very high melting point with low-solvent solubility.
Robert Hoerr: Intractable compounds are typically very poorly soluble and also poorly permeable (i.e. Class IV compounds.) Strategies for oral administration often require unique (and expensive) technologies and often use alternative delivery methods, such as intravenous administration. In the case where delivering a Class IV compound orally is the goal, the improved solubility at the absorptive surface that an amorphous solid provides may be insufficient to generate the required permeability. Nanoformulation, such as nanocrystal suspensions, has provided some improvement in bioavailability. The ENS approach could be extended to BCS class IV drugs by spray excipient combinations that encapsulate or otherwise modify surface chemistry to facilitate permeation.
Keith Hutchison: Occasionally, a high dose, poorly lipid soluble, low permeability compound comes along that would be a typical intractable compound. We also consider the biopharmaceutical aspects of the API, such as rate of aborportion and clearance, P-gp efflux potential, and lymphatic drug transport – these API characteristics further guide our formulation design.
Dave Miller: An intractable compound for the KinetiSol process would be one that is extremely thermally unstable at elevated temperatures in its amorphous form, ie, rapidly degrades in seconds at elevated temperatures. Also, compounds that are chemically unstable in the amorphous form at common storage conditions can be very difficult to manage with KinetiSol, as with all amorphous dispersion technologies.
Peter Nelson: There are a few physical and chemical properties that would make an API “intractable” for micronization. For example, an API that will not readily fracture would not be a good candidate for micronization. We have seen very few APIs, however, that are not able to be reduced in size. The question becomes whether or not the size attainable via micronization produces the desired results. Any API that has been synthesized to a size consistent with “micronized” powder (ie, less than 10 microns) could present a challenge for further size reduction. Compounds with low melting points can be considered “intractable” where mechanical milling (eg, pin-milling or hammer-milling) is required. Mechanical milling may produce sufficient heat to melt the API. Hydrates may also be challenging to work with in cases where increased surface area produces API that is overly susceptible to moisture loss under low relative humidity. This becomes less of a concern when the micronized product is exposed to adequate moisture and permitted to rehydrate. In most cases, there are ways to address the physical and/or chemical challenges associated with most APIs. Ultimately, the decision to micronize is based on whether or not the increase in surface area produces the desired level of bioavailability.
URBAN MYTHS
Q: Are there urban myths about when to use or to avoid a specific solubilization technology that diminish opportunities for solubilization?
Dan Dobry: In the past, it was common to hear concerns about formulations relying on amorphous APIs. In particular, there was a great concern about physical stability and the need for what were perceived as new technologies, such as extrusion, spraylayered coating, or spray-drying. With the commercialization of compounds, such as Kaletra, Incivek, Zelboraf, Kalydeco, and other amorphous APIs, it has become accepted to use non-crystalline approaches for delivering low-solubility compounds. Amorphous physical stability prediction has come a long way, including use of predictive tools as an approach to de-risking long-term stability studies. It’s possible to accurately predict worst-case stability using rapid measurement techniques in combination with theory on the fragility of glasses. Bend Research scientists are happy to describe the methodology, case studies, and theory for kinetically stable glasses with those interested. Understanding the role that processing plays in creating these stable forms is also much better understood now – especially using spray-drying.
Tom Dürig: Frequently, when a compound has solubility-limited absorption, nano-crystalline material is of limited use because it does not affect API solubility. For a compound with strong crystal-lattice energy and medium-to-low log P, lipid formulations are often not a very effective solubilization approach. For very high log P and/or high crystal-lattice energy APIs, solid dispersions tend to suffer from low drug loads.
Joan Feixas: Until recently, the pharmaceutical cocrystal approach has not been a preferred option in the pharmaceutical industry because there are no marketed drugs formally approved as cocrystals. However, the reality is that some drugs that were considered to be salts when approved, turned out to be cocrystals. The interest in cocrystals is clearly growing as evidenced by the increase each year of the cocrystal patent number. Today, there are a number of pharmaceutical cocrystals progressing through clinical trials, and it is only a matter of time before some of these will be commercial. Then, we will be able to speak of “official” pharmaceutical cocrystals.
Filipe Gaspar: There is the misperception that spray-drying is not adequate for processing thermal labile drugs or that the low yields at bench scale are representative of the technology. In fact, the technology is very gentle from a thermal viewpoint, and any reasonably developed process, at the right scale, delivers yields of at least 90%, more often 95% to 98%. For HME, it is commonly supposed that the technology cannot be used in high melting point drugs, but in some cases, dissolution in the polymer occurs at lower temperatures. Robert Hoerr: Historically, amorphous forms of API have been deemed thermally unstable and therefore unsuitable for meeting environmental and shelf-life requirements. The flexibility of electrospray technology can provide highly improved, homogeneous mixing of drug and excipient in varying ratios. Multiple streams allow for a wide range of ratios that can include nanosized or molecular subcomponent in solid dispersions or solid solutions. Targeted ratios can show improved solubility, suspendability, and dispersability while not compromising or even improving thermal stability.
Keith Hutchison: A common myth is that each solubilization technology is mutually exclusive whereas in fact there is sometimes considerable overlap between different technologies. For example, an API produced in a nanoparticle form and also solubilized in a lipid-based formulation may show similar bioavailability in fasted subjects; however, if the API has a positive food effect, the lipid formula is most likely to overcome this particular issue in a fed study. In addition, a high log P is not always necessary to produce a lipid formulation (though it might help) because a less lipophilic API can still be solubilized in a lipid-based formulation, particularly if the compound possesses a low melting temperature and/or is low dose.
Dave Miller: There is a common misconception or exaggeration regarding the inherent instability of amorphous formulation systems. While it is true that for most compounds, the neat amorphous API is physically unstable/metastable and will eventually convert to the lower energy crystalline state, this is not true for properly designed amorphous solid dispersion systems. By utilizing appropriate in silico and empirical tools, one can design an amorphous solid dispersion formulation that is either thermodynamically stable, or is kinetically stable for a period of years at typical storage conditions. The anxiety regarding the myth of inherently unstable amorphous systems has very likely limited the application of amorphous dispersion technologies for developing compounds whose therapeutic potential may not have been realized as a result.
Peter Nelson: A common urban myth related to micronization suggests that the micronization process produces excessive amorphous content and may lead to crystalline phase transitions. While some level of amorphous content may be generated under aggressive milling conditions, the % amorphous content is typically very low. Our own testing of hundreds of micronized APIs via XRPD, DSC, and other techniques indicates that true crystalline phase transitions seldom occur as a result of the micronization process.
Deepak Tiwari:We occasionally see nanoparticles as a class being ruled out because one or another approach failed. Nanotechnologies are diverse with different manufacturing processes and mechanisms of stabilization, so failure in one has little bearing on the chances of another.
THE NEXT ISSUE
In Part 2, we’ll hear more from solubilization technologies experts, including innovative ways to leverage and combine technologies to expand opportunities for overcoming poor solubility. To ensure The Second Quadrant serves as a forum for interactivity and collaboration, I invite you to send your reactions, thoughts, and suggestions so we can continue our dialogue. I look forward to hearing from you.
REFERENCES
1. Butler J. Product Development, GlaxoSmithKline. The optimal use of biorelevant media & simple modeling for the prediction of in vivo oral behavior. The Academy of Pharmaceutical Sciences Event. June 9, 2011.
Total Page Views: 5386