Issue:March 2014
BIOAVAILABILITY ENHANCEMENT - Analysis of the Historical Use of Solubilization Technologies
In 2013, contributors to The Second Quadrant gave insights into how to decide what solubilization technologies might be most appropriate based on the API’s characteristics and dosage form requirements for the drug product. Pharmaceutical companies are increasingly focusing on the use of solubilization to advance their most promising compounds to the marketplace. The Developability Classification System (DCS) further refines the FDA Biopharmaceutics Classification System (BCS), overlaying dissolution rate onto solubility and permeability and providing a third dimension to assist in categorizing an API and understanding what type of solubilization strategy is most likely to succeed.
The progress being made in the knowledge base around addressing BCS Type II compounds has been significant in the past decade, and appears to be accelerating. In this month’s column, we take a historical look at the marketed drugs that have been solubilized, the technologies used, and the therapeutic areas these drugs address. The goal of this analysis is to gain better insight into the commercial use of solubilization and its impact on the pharmaceutical industry.
THE GROWING USE SOLUBILIZATION TECHNOLOGIES SINCE 1975
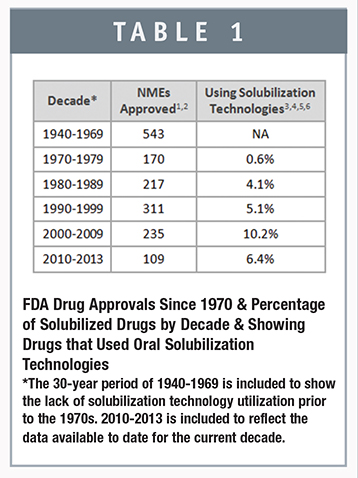
Since 1975, we have found that approximately 60 marketed drugs have leveraged solubilization technologies to enhance oral bioavailability. In the preceding 36 years, from the time the FDA required submission of an NDA in 1938, solubilization technology was virtually unused on a regular basis. Apparently, the disease areas focus, drug discovery methodologies, and the lack of mature solubilization platforms restricted the use prior to the 1970s. In comparison, the past nearly 4 decades have shown robust growth in the reliance on solubilization platforms, accounting on average for around 6% of all NMEs approved from 1975 through 2013, and more than 10% in the past decade (Table 1).1-7 Some years stand out to validate the need and use of solubilization platforms. For example, in 2005, 20% of NMEs approved used technologies including solid dispersion, lipid, and nanocrystal platforms. The data for the most recent 4-year period (2010-2013) seems to represent a slight decline in growth, but it is still early in the decade, and the data set is relatively small. Based on the trends throughout the past 4 decades and the changing chemical space in drug development, we expect the decade will show additional and significant current growth in use of solubilization technologies once we have visibility into the full 10-year period.
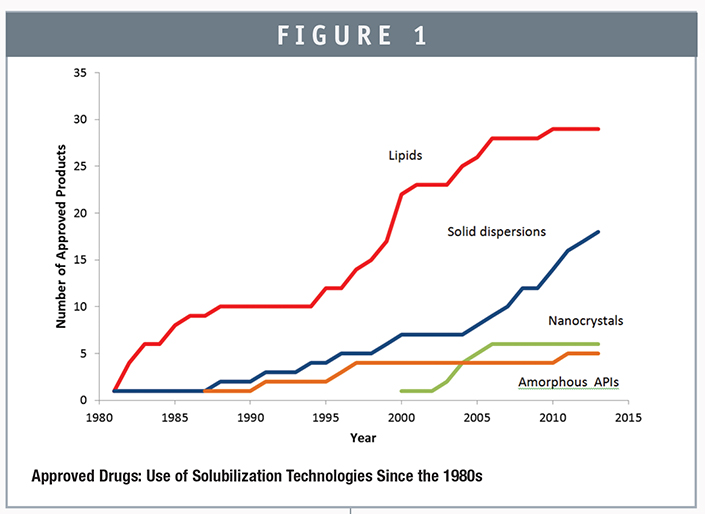
SOLUBILIZATION TECHNOLOGIES: BY MARKETED DRUG APPROVALS BY DECADE
The popularity, utilization, and success of the diverse technologies throughout the past 6 decades is reflected in changing landscape of approved drugs applying solubilization approaches since the 1970s. In order to gain a quantitative understanding of the historical role of solubilization, we have compiled a comprehensive database of approved drugs that are formulated using a variety of solubilization technologies and delivered orally. In addition, we further filtered the dataset to only include formulations delivered as tablets or capsules (excluding solution and suspension vehicles). The technologies that have been used were divided into four classes: solid dispersions, pure amorphous APIs, lipids, and nanocrystals. From our analysis, we confirm that lipid technologies have the most widespread use in terms of drug approvals in the years prior to 2005. The use of solid dispersion technologies has also seen strong growth but has lagged lipids by approximately 5 to 7 years. However, it appears that throughout the past decade, the growth rate of solid dispersions has been twice that of the lipid formulations. While it is not possible to determine the reasons in the available data, the more rapid adoption of solid dispersions may be a result of many factors, including the attractiveness of a tablet dosage form (and conventional processing equipment), generally higher unit dose achievable, the widespread availability of manufacturing capabilities (HME and spray-drying), and the exponential growth in scientific knowledge of solid dispersions in the past decade as reported in last month’s column.3-6
TECHNOLOGIES USED & THERAPEUTIC AREAS ADDRESSED
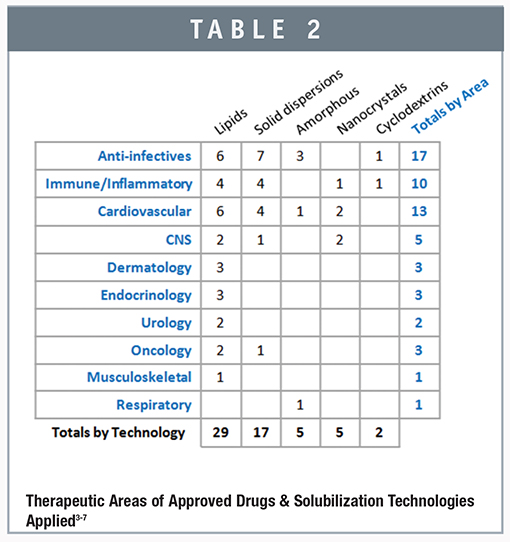
The PhrMA 2013 Profile of the Biopharmaceutical Industry points to a study finding that since 1975, medicines have contributed to a 60% increase in survival rates of cancer patients, and that research done by the American Heart Association has found that in the 10-year period of 1999-2009, death rates due to cardiovascular disease have dropped by 33%. Even more dramatically, since the approval of antiretroviral treatments in 1995 the HIV/AIDS death-rate has dropped by 85%. We performed a study to evaluate the role solubilization technologies play to support drug success in the various therapeutic areas, and the findings were surprising. Table 2 shows the number of commercial products that are in the various delivery platforms and in each therapeutic area. The industry has long been aware that there’s a strong need for solubilization in the drugs addressing the therapeutic areas of anti-infectives, cardiovascular, and immune/inflammatory. One surprising observation that this analysis reveals is the relatively few applications of solubilization to oncology. This is surprising to us at Agere since a large proportion of the compounds in our development portfolio are in oncology. One explanation for this may be that the approach to treating cancer has shifted from cytotoxic agents to targeted therapies, and these compounds, which are largely still in development, have lower solubility in general. In addition, many oncology drugs in the past have been dosed using methods other than oral delivery and as solubilization technologies have improved significantly, more therapeutic agents are now being developed for oral use.
CONCLUSIONS
This analysis covers the drugs that have been approved, and the data suggests a strong and increasing adoption of various technologies – especially throughout the past 15 years. What lies ahead for the rest of the current decade? The combination of three key factors – the complexity of diseases being addressed, that modern drug targets favor compounds with poor solubility and today’s methods for designing, synthesizing and optimizing chemical libraries – promise to increase the reliance on solubilization technologies to realize the potential of promising drugs in development. It’s a safe prediction that the utilization of bioavailability-enhancing platforms will rapidly accelerate before the end of the decade.
To view this issue and all back issues online, please visit www.drug-dev.com.
REFERENCES/NOTES
1. FDA, Summary of NDA Approvals and Receipts.1938-present.
2. FDA New Molecular Entity Approvals. 2012-2013.
3. Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS. DrugBank 3.0: a comprehensive resource for ‘omics’ research on drugs. Nucleic Acids Res. 2011;Jan;39(Database issue):D1035-41. PMID: 21059682.
4. Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;Jan;36(Database issue):D901-6. PMID: 18048412.
5. Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;Jan 1;34(Database issue):D668-72. PMID: 16381955.
6. Agere analysis.
7. This estimate is thought to be conservative, as drugs thought to have used solubilization technologies but that were not yet verified were not included in this calculation.
Total Page Views: 2946