Issue:January/February 2020
AUTOINJECTOR PLATFORM - Identifying New, Enhanced Device Delivery Solutions for Chronic Diseases
ABSTRACT
Biologics now constitute a significant element of available medical treatments. Owing to their clinical and commercial success, biologics are a rapidly growing class and have become a dominant therapeutic modality.1 This, and the increased demand for home healthcare, driven by the ever-increasing prevalence of chronic diseases, is fueling increased demand for self-injection devices.2
For manufacturers, while many are turning their attention to devices that support injection of higher volumes and greater viscosities, there remains a need to further innovate in the 1-mL space and provide innovative injection solutions for viscous drugs in the 2-mL space to improve delivery outcomes, patient quality of life, and adherence to treatment.
Reducing injection frequency is one of the strategies implemented by pharmaceutical companies. To reach this objective, they will be looking to increase their drug dosage and potency, either by increasing the drug concentration and/or delivery of larger volumes.3,4 Needle innovations, including the usage of shorter needles with ultra-thin wall technology for prefilled syringes, supports the delivery of drugs with greater volumes and viscosities, which greatly enhances end-user experience.
CHRONIC DISEASE MARKET, SELF-INJECTION DEVICES
Self-injection devices are designed for multiple injections of biologics and hormones for patients requiring frequent dosage for the long-term management of medical conditions, such as diabetes and rheumatoid arthritis. As the population ages, the incidence of these diseases increases.
Today, more than 70 million Americans aged 50 and older – four out of five older adults – suffer from at least one chronic condition, while 11 million live with five or more chronic conditions.5 As the population ages, the prevalence of chronic diseases will increase. In the US, these persistent conditions are the nation’s leading cause of death and morbidity.6
Globally, chronic diseases have affected the health and quality of life of many citizens. In addition, chronic diseases, and poorly managed chronic diseases have important societal costs as they depress wages, workforce participation and labour productivity, as well as increase early retirement, high job turnover, and disability.7 Poorly managed chronic diseases have an even greater impact. The increase in the incidence of chronic conditions, as well as the increase in home healthcare, are two of the key drivers of growth for drug injection delivery solutions. In the attempt of improving self-injection experience for chronic disease patients in the home setting, pharmaceutical companies often develop self-injecting devices combining primary container (eg, prefilled syringes) with needle stick safety guard or autoinjector solutions. These are usually administered by a skilled medical professional, a formal or informal caregiver, or by the patients themselves.
The use of a drug delivery device will improve patient concordance and persistence to treatment, and it can reduce the total cost to payers. For drug delivery manufacturers, a relentless focus on patient centricity helps customers enhance patient injection experience, ease of use of the drug delivery solution, and patient adherence.
PATIENT-CENTRIC PRODUCT DEVELOPMENT
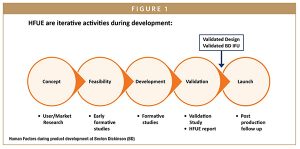
Human factor engineering has become an integral part of the product development cycle. As a result, human factor considerations are playing an ever-increasing role as innovative drug delivery systems are coming to market (Figure 1). The goal of product development should be the design of robust drug delivery systems that are effective and easy for patients to self-administer every time. Healthcare professionals, patients, and caregivers need to safely prepare, place, activate, and remove the device with minimal difficulty. Human factor engineering should therefore be adopted to improve safety, reduce risk, ensure ease of use, and verify that a product design will be effective.
For manufacturers, human centered design principles should be at the heart of development of drug delivery devices. Usability should be considered throughout the development process, starting with design input, to ensure product effectiveness, efficiency, and user satisfaction in the intended use environment. The aim is to minimize user-related risks and improve user adherence by checking on ease of use, patient comfort, concordance, and persistence, and by providing easy instructions and solutions for patients with limited dexterity and reduced eye sight.
A patient-centered design approach combines clinical data, usability engineering, and patient insights that meet the needs of customers and their patients. Increasing the focus on patient centricity enables patients greater freedom to live their lives without being restricted by their drug delivery regimen.
ADDRESSING THE TREND TOWARD MORE VISCOUS DRUGS
There is an increasing trend for viscous biologic drugs to be developed in the chronic disease space. As a consequence, there is a high need for performant and robust drug delivery solutions that can administer highly viscous drugs to improve delivery outcomes and patient adherence to treatment.
The actual biotech market is driven by the 1-mL subcutaneous injection delivery solution, where there is a high need to increase drug potency and thus reduce injection frequency. To reach this objective, biotech companies will be looking to increase their drug concentration, which in turn can lead to exponential increase in viscosity. When viscosity becomes very high, they might consider reformulating the drug in a higher volume. Consequently, today, we can see more drugs developed in a 2-mL volume. To administer these viscous drugs within an acceptable injection time that a patient can tolerate – approximately between 10 and 15 seconds – there is a need for a reliably performing and robust delivery solution, especially when these drugs are administered with manual delivery needle standards, as pharmaceutical companies may not want to compromise on patient injection experience.
INNOVATIVE SHORT NEEDLE TECHNOLOGY
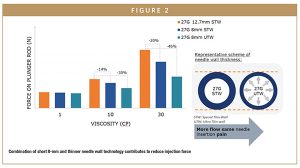
Standard needles for chronic disease treatment and administration are historically 12.7 mm long. Reducing needle length to 8 mm enables the delivery of larger, more viscous drugs, while enhancing end-user injection experience. Moreover, the risk of reaching the intramuscular (IM) layer during a subcutaneous (SC) injection is considerably reduced with an 8-mm exposed needle length versus 12.7-mm needles without increasing the risk of accidental intradermal (ID) injection, which in turn could lead to adverse immune responses.8 This risk reduction is especially important in the absence of a skin pinch, which is usually recommended with the 12.7-mm standard needle, or when done with populations with thin SC fat or with children (Figure 2).
To address the specific needs of pharmaceutical companies developing viscous high-volume biologics, BD has launched a short needle technology based on its advanced BD NeopakTM 2.25-mL glass prefillable syringe platform, which is available with 12.7-mm and 8-mm needle solutions (Figure 3).
Another way to improve the performance of viscous drug delivery without increasing injection force is to work around critical needle parameters, such as needle inner diameter. To address this, BD is developing a prefilled syringe solution combining shorter needle length (8 mm) with ultra-thin wall (UTW) needle technology for use in combination products. This technology increases the inner diameter of the needle by reducing the thickness of the needle wall without having to increase the external diameter (Figure 2). The outer diameter of a needle is known to be an important feature as it influences needle insertion pain perception and the level of injection-related anxiety experienced by patients.9 Mathematical simulations show that using an UTW needle can reduce injection force by 46% for highly viscous solutions (Figure 2). This innovation combining short 8-mm exposed needle with UTW technology therefore reduces the injection force, especially for viscous solutions, without compromising on needle robustness, insertion pain, and pain perception.
In order to further enhance patient experience and improve compliance to treatment, pharmaceutical companies will often integrate an autoinjector to the manual delivery container. However, it is increasingly difficult to develop a single designed autoinjector that can effectively and safely inject the variety of parenteral drugs with different viscosities developed by a single pharmaceutical company.
What we see today in the market are autoinjectors designed and customized to target a narrower range of drug viscosities. So effectively, multiple autoinjector devices are being used to deliver different drugs by one single pharma company. There is therefore a need for biotech companies to have a “platform” autoinjector device that can deliver a wide-range of viscosity drugs without customizing the system components.
INTRODUCING BD INTEVIATM AUTOINJECTORS
Becton Dickinson has recently launched InteviaTM 1-mL autoinjector, a platform technology solution intended for use in a home setting by patients living with auto-immune and chronic conditions. The platform provides a new solution for drug manufacturers that not only supports patients, but it supports the efficiency of the manufacturing process itself.
InteviaTM autoinjector platform includes a 1-mL and a 2.25-mL version (Figure 4). They are both two-step push-on-skin autoinjectors that are designed to effectively and safely inject a variety of drugs of different range of viscosities up to 35 cP and different fill volumes, without customizing the components.
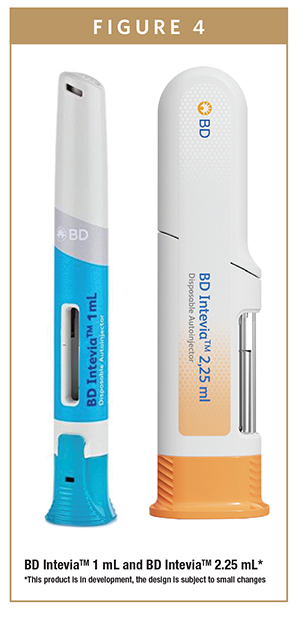
In terms of patient centricity, the InteviaTM platform enables ease of use and has a patient-friendly design, while its unique feedback indicator and audible click provide assurance to patients when the correct dose has been delivered.
InteviaTM system integration with prefilled syringes reduces the risk of dosing errors, drug wastage, and needlestick injuries. Post-injection, the needle is protected, further safeguarding patients and caregivers. Indeed, all of its features provide patients with the confidence and convenience to self-inject as prescribed by their doctor.
The InteviaTM platform is the only push-on-skin autoinjector that is designed with proprietary knowledge of primary container process controls, integrating all the functionalities to maximize product performance. It is a well-integrated system that ensures robust performance to help manage development process risks and optimize time to market of combination products.
BIOTECH MARKET, DRIVEN BY 1-ML DELIVERY SOLUTIONS
The biotech market is driven by the 1-mL subcutaneous injection delivery solution and typical autoinjectors available on the market use a 1-mL syringe. However, many of these are not integrated platforms, and this provides some system integrated challenges for pharmaceutical companies. BD’s new injection device helps overcome these.
In designing a robust and performing autoinjector, device manufacturers need to ensure a high compatibility between biotech drug, primary container, and secondary packaging. To ensure that, there is a need to master the engineering process and generate a robust set of data that bring the primary container (eg, prefilled syringes) and the secondary packaging (delivery systems) co-operating together in one performing system that will safely and effectively deliver the drug to patients, with reproducible performance across millions of units.
Becton Dickinson has developed deep domain knowledge to support the development of more robust, well-designed systems, such as the new InteviaTM platform. This means that biotech companies have a “platform” autoinjector device with the potential benefits this offers in terms of manufacturing efficiency and time to market. To offer a seamless integration, BD has designed its latest BD InteviaTM 2.25-mL large-volume autoinjector around its latest UTW 8-mm needle technology. This combination will allow pharmaceutical companies to push the boundaries of viscosities further and enhance patient injection experience.
SUMMARY
In addition to wider uptake of home care, an increase in the number of biologics being manufactured, many by the same company, is driving ongoing innovation in drug delivery systems. While the ultimate aim is to improve patient experience and health outcomes, innovation can also offer the pharmaceutical industry savings in terms of assembly costs and time to market.
Identifying enhanced needle solutions and offering integrated system solutions to serve the chronic disease market is a great step toward addressing improved patient outcomes and adherence. The InteviaTM platform sits among BD’s vast portfolio of drug delivery solutions and affords this opportunity as it can accommodate different fill volumes and different level of viscosities without having to change any component in the delivery system. As the market further develops over the coming years, we should expect to see further innovations to meet the growing need for self-injection devices.
REFERENCES
- Anselmo C, Gokarn Y, Mitragotri S. Noninvasive delivery strategies for biologics. Nature Reviews Drug Discovery. 2019;18:19-40.
- Transparency Market Research. Available online: https://www.transparencymarketresearch.com/self-injection-devicemarket.html. (Last accessed: November 2019).
- Agrawal N, Helk B, Kumar S et al. Computational tool for the early screening of monoclonal antibodies for their viscosities. mABs. 2016;8(1):43-48.
- Yang Y, Velayudhan A, Thornhill et al. Multi-Criteria Manufacturability Indices for Ranking High-Concentration Monoclonal Antibody Formulations. Biotechnology and Bioengineering. 2017;114(9):2043-2055.
- American Association of Retired Persons. Chronic Conditions among Older Americans. Available online: https://assets.aarp.org/rgcenter/health/beyond_50_hcr_conditions.pdf (Last accessed: November 2019).
- Comlossy, M. Chronic Disease Prevention and Management. National Conference of State Legislatures: Denver, CO, USA, 2013.
- Raghupathi W and Raghupathi V. An Empirical Study of Chronic Diseases in the United States: A Visual Analytics Approach to Public Health. International Journal of Environmental Research and Public Health. 2018; 15:431.
- Baker M, Reynolds H, Lumicisi B et al. Immunogenicity of protein therapeutics. The key causes, consequences and challenges. Self/Nonself. 2010;1(4):314-322.
- Usach I, Martinez R, Festini T et al. Subcutaneous Injection of Drugs: Literature Review of Factors Influencing Pain Sensation at the Injection Site. Adv Ther. 2019;36:2986-2996.
To view this issue and all back issues online, please visit www.drug-dev.com.

Karima Yadi is the Global Marketing Lead for the autoinjector platform at BD Medical-Pharmaceutical Systems. She provides commercial leadership to BD’s delivery system platforms and defines, develops, and launches patient-centered injection solutions in collaboration with cross functional, commercial, and regional teams. She has held different roles of increasing responsibility within Pfizer Inc. in Marketing and Business Development and at Zoetis in Regional Marketing in the Companion Animal Business Unit. She has deep experience in product launches and product life cycle management, from the development phase to the commercialization phase. She earned her BSc (Hons) in Management Science from University of Warwick Business School, and her MSc in International Business Management from London South Bank University.

Lionel Maritan is R&D Associate Director at BD, responsible for Design, Development, and Life Cycle Management activities for Autoinjectors and Safety solutions. He joined BD in 2005 and held roles of increasing responsibilities within R&D. He has deep experience in Drug Delivery Systems Design and Development from the innovation stage to commercialization. He developed, in particular, the BD PhysiojectTM Autoinjector and is listed on many patents. Prior to BD, he worked in the automotive industry. He has a MSc in Engineering with a specialization in plastic parts design and manufacturing.
Total Page Views: 8337