2019 Analytical Testing in Drug Development eBook - Regulations Help Propel Testing Market
The global pharmaceutical analytical testing outsourcing market is expected to reach a valuation of almost $8.1 billion by 2023.1 The expected swell in analytical tests, including bioanalytical testing, method development and validation, stability testing, and extractables and leachables testing, is being attributed to, among other things, increasing regulation.
As an example, the FDA updated its guidelines on opioid formulations to employ strategies to reduce or eliminate the potential for abuse and misuse. The agency requires performing a scientifically rigorous laboratory-based in vitro manipulation and extraction study to evaluate how easily the product can be abused or compromised. Abuse-deterrent studies involve various types of physical manipulation, extractability and solubility studies, complex analytical methodology, and particle size characterization. Not only are these necessary for NDA and ANDA filings, but they may help reduce the scope and cost of subsequent pharmacokinetic and clinical studies.
Additionally, as regulatory bodies like the International Council for Harmonization place method lifecycle management (MLCM) at the forefront of future regulation, many CDMOs are adopting MLCM. The technique creates a framework for defining the principles of analytical methods in a more structured way. The continuous process documents the capabilities of each analytical method used in the clinical development of a potential new drug, and its adoption ensures the quality of pharmaceutical products.2
Regulation was also discussed at the recent Extractables and Leachables USA 2019 conference. With the release of ISO 10993-1:2018 and the revision to ISO 10993-18, chemical characterization testing has skyrocketed to the forefront of necessary information for toxicological evaluation of medical devices. With increased scrutiny on the necessary threshold limits needed for chemistry data, the study design for an effective extractables characterization must include careful assessment of the extraction solvents and the anticipated material interactions.3 Studying the impact of extractable and leachable substances on safety and drug product interaction is of utmost importance to protect the patient and comply with regulations.
Finally, FDA’s guidance on process analytical technologies (PAT) provides a framework for pharmaceutical development, manufacturing, and quality assurance. The global market for PAT in pharmaceuticals should reach nearly $540 million by 2022, up from $412.7 million in 2017, according to BCC Research. PAT, developed for pharmaceuticals, has a broader scope and reaches into biotechnology and biopharmaceuticals. PAT includes chromatographic tools and techniques, spectrophotometric tools and techniques, capillary electrophoresis, titration tools, and automated analytical methods. According to BCC Research, by 2022, spectrophotometric tools could reach $242 million and automated analytical methods $68.5 million.4
This Drug Development & Delivery Analytical Testing in Drug Development eBook showcases the various types of services that leading testing companies offer and how they are helping industry comply with regulations to get their products approved and commercialized.
References
1. Pharmaceutical Analytical Testing Outsourcing Market is expected to register a CAGR of 8.6% to reach USD 8,095.0 million till 2023, Says Market Research Future, Market Research Future Reports, May 29, 2019, https://www.globenewswire.com/newsrelease/2019/05/29/1856453/0/en/Pharmaceutical-Analytical-
Testing-Outsourcing-Market-is-expected-to-register-a-CAGR-of-8-6-to-reach-USD-8-095-0-million-till-2023-Says-Market-Research-Future.html.
2. A Better Lifecycle for Analytical Methods? Technology Networks, Ruairi J Mackenzie, May 20, 2019,
https://www.technologynetworks.com/informatics/blog/a-better-lifefor-analytical-methods-319591.
3. Extractables and Leachables USA 2019,https://www.namsa.com/events/extractables-leachables-usa-2019/.
4. Process Analytical Technologies for Pharmaceuticals: Global Markets, BCC Research, Shalini Shahani Dewan, January 2018, https://cdn2.hubspot.net/hubfs/308401/IAS_Report_Overviews/IAS034C_Report_Overview.pdf?utm_campaign=IAS034C&utm_source=hs_automation&utm_medium=email&utm_content=593
77623&_hsenc=p2ANqtz-9slaGMb9gkVeMwIQ01x6rC1E_tzTvYiyGZPfJIOBbgrn1XKWEdXwxN
v6zsXneVSvI2Ab8byYbkT3J_X4G8epz2tPHeLw&_hsmi=59377623.
Considerations for Abuse-Deterrent Category 1 Studies
 Angela Moore, MSc, Alcami Scientist, has considerable experience performing analytical testing in the pharmaceutical industry for both branded and generic products and their active pharmaceutical ingredients; she has successfully executed several FDA Category 1 in vitro abuse-deterrent studies on drug products that are designed to help combat opioid abuse.
Angela Moore, MSc, Alcami Scientist, has considerable experience performing analytical testing in the pharmaceutical industry for both branded and generic products and their active pharmaceutical ingredients; she has successfully executed several FDA Category 1 in vitro abuse-deterrent studies on drug products that are designed to help combat opioid abuse.
Healthcare professionals around the world are commonly prescribing opioid medications for pain management, generating an inevitable association with abuse and addiction. The Centers for Disease Control and Prevention (CDC) has estimated that, in 2017, over 47,600 Americans died from opioid overdoses. Of these deaths, 35% involved the use of prescription opioid medications.*
Government officials and pharmaceutical professionals alike are in need of risk mitigation approaches. The Abuse Deterrent Access Act of 2018 requires Medicare and Medicaid to report to Congress the availability of abuse-deterrent opioid pain treatments. Pharmaceutical companies have responded to this need through more diligent and extensive abuse-deterrent formulations and studies.
Current US Food and Drug Administration (FDA) guidelines for determining the effectiveness of abuse deterrence for a drug substance involve four main studies termed Category 1, 2, 3, and 4. Category 1 studies are performed analytically, Category 2 and 3 studies are performed in vivo, and Category 4 assess long-term impact.
Category 1 Studies
“In these (Category 1) studies, the product is evaluated and compared to currently marketed formulations for the ability to defeat or compromise the abuse-deterrent properties. This testing is done in vitro and evaluates the drug’s ability to resist crushing, grinding, melting, etc. to limit nasal abuse. Extraction studies provide information about the product’s ability to isolate the antagonist, resist abuse by injection, or, in larger volumes, diminish abuse by ingestion,” says Angela Moore, MSc, Head of Alcami’s Abuse-Deterrent Studies Program. “One of the most important aspects of Category 1 testing is study design and repeatability. Multiple replicates give confidence in results. Also, a well-thought out Design of Experiment and testing to failure is vital for these studies. Exact study requirements are not clearly defined through current guidance and the FDA uses a ‘totality of evidence’ approach when evaluating these formulations.” With these studies, it is necessary to represent the worst-case scenario — always try to test a product to failure. “If a product is tested to failure and shows that it’s better than the comparators under the worst situations for your product, you have a compelling story to tell the FDA,” states Moore.
When conducting Category 1 abuse-deterrent testing, typical studies mimic real-world abuse of the opioid in question. The route of drug abuse depends on the person’s personal preference; routes of abuse include oral, insufflation, injection, and smoking. Key examples of Category 1 testing are physical manipulation evaluations, large volume extractions, syringeability testing, smoking studies, liquid-liquid, free-base extractions, and, if needed, isolation of opioid/antagonist studies. One critical evaluation that can only be done in vitro are syringeability studies.
Standard Syringeability Studies
According to the 2015 FDA guidance for the industry, “the amount of opioid that can be obtained in a syringe should be based on studies of intact and manipulated test product and comparators using small volumes of water (5-10 mL) at room temperature and at 90-95°C with and without agitation (Administration, 2015).” The FDA also advises from the 2017 FDA generic guidance: “10mL volume, 5-60 minute extraction times, and needle gauge 21 or finer (US Food and Drug Administration, 2017).”
Moore states: “Unlike common dissolution studies or large-volume extraction studies, when performing syringeability studies in the laboratory, an individual sample must be prepared for each time point evaluated. This is due to the large amount of data needed for each individual sample. In vitro syringeability studies are critical because these studies are not performed or verified in humans. The evaluation of a formulation for syringeability abuse solely relies on laboratory data.
In conclusion, abuse-deterrent medications can not only be a competitive advantage, but they save lives. When designing Category 1 testing, it is best to anticipate what drug users will do to manipulate the drug and test to failure. Alcami specializes in Category 1 studies, has full GMP traceability, and uses a team-based approach when applying appropriate resources to effectively utilize and meet client deadlines. This is especially important as Category 1 strategies and results drive the in vivo Category 2 and 3 studies required for abuse-deterrent labeling of drug products.
References
1. US Food and Drug Administration. (2015). Abuse-Deterrent Opioids — Evaluation and Labeling Guidance for Industry.
2. US Food and Drug Administration. (2017). General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products Guidance for Industry.
*An age-adjusted increase of 9.6% from 2016 according to the Centers for Disease and Prevention (CDC)
Advances in Analytical Testing Within the Burgeoning Biologics Business

Franck A. Vendeix, PhD, is Associate Director, Analytical Development and works as an expert scientific consultant for the Analytical Development Group at AMRI.
With patent expirations fueling a boom in biosimilars and research in areas such as gene therapy, the biologics market is expected to be worth an estimated $479.7 billion by 2024.1
In such a competitive environment, high-quality analytical testing that speeds and simplifies the journey from bench side to bedside is critical.
Industry-leading biochemistry experts are rising to the challenge by utilizing state-of-the art technologies, such as ultrahigh-resolution mass spectrometry (MS) coupled with capillary electrophoresis (CE). These methods used standalone, or in tandem, provide highly accurate chemical information that roots out process impurities, contaminants, and degradants, which constitute a paradigm shift in sample separation and analytics.
Analytical Services
Biochemical analytical services provide cGMP support for all stages of biopharmaceutical development and manufacturing. Specialist services include method development, validation and transfer, cGMP batch release testing, impurity characterization and identification, and drug substance, product stability, and comparability studies. More sophisticated means of analysis require state-of-the-art tools in the hands of industry-leading expertise.
Next-Generation Mass Spectrometry
The modern Q-TOF mass spectrometer has significantly improved analysis and data interpretation of small and large molecules, including biologic drugs, metabolites, and polymers. Modern hardware and software are also compliant with ICH Q6B specifications for Biotechnological/Biological Products.
These systems can unambiguously assign molecular formulae, and the mass resolving power enables analyses in the presence of complex biological and chemical matrices.
HRMS identifies levels of active and/or high-percentage metabolites and analytes in drugs, complying with FDA Metabolites in Safety Testing (MIST) guidance, and providing safety information early on. Its enhanced sensitivity, with a limit of detection in the pg/mL to fg/mL range, is particularly important when performing impurity assays.
HRMS has an extreme mass range 20 Da to 40,000 Da, assuming its working with singly charged ions. This enables the analysis of small molecules, polymers, carbohydrates, oligonucleotides, peptides, and proteins. Far larger masses can be analyzed if multiple charges are present.
In liquid chromatography-mass spectrometry (LC-MS), the system is compatible with ultra-performance liquid chromatography (UPLC), which is five to 10 times faster than high-performance liquid chromatography (HPLC), with two to three times the chromatographic resolution. This results in easier method development and a more rapid turnaround on larger numbers of samples.
In addition to standard UV detection in UPLC, state-of-the-art charged aerosol detection (CAD) is available, and Q-TOF’s tandem MS (MS/MS) capabilities allow teams to get the most information from a limited amount of sample. It can also be used to sequence proteins from the N-terminus or C-terminus. And when MS is skillfully combined with the high separation power of CE, researchers can expect even more sensitive detection and more detailed content information.
CE: The Future of Sample Separation
There is an increasing demand for the analysis of chiral molecules, and polymers and macromolecules from mixtures. Traditionally, large molecules analyses (proteins, oligonucleotides) have been carried out using Gel Electrophoresis (GE). But it can take anything from a few hours to a few days to set up, and the results show only a very low resolution of the separations and are difficult to quantify.
SDS-PAGE devices are not compliant with Title 21 of the FDA’s Code of Federal Regulations Part 11, and do not have the temperature control needed to provide sample stability.
CE is a game changer, cutting separation time to just five or ten minutes and requiring only small samples, making it beneficial when analyzing rare or expensive compounds.
Powerful CE instruments can separate proteins, nucleic acids or other molecules from mixtures with high levels of accuracy, meaning it has a role in areas including genetic sequencing and gene therapy. It’s useful in single-nucleotide polymorphism (SNP) analysis, DNA/RNA fingerprinting, and impurity quantification – which it can measure to 0.01%. What’s more, the higher voltage possible with capillary tubes enables single-nucleotide resolution, enabling molecular weight (MW) and purity analysis.
Combining the power of mass spectrometry and capillary electrophoresis techniques and expert interpretation provides sensitive biologics separations with high resolution mass analysis.
CE-MS as a Solution to Competing Demands
Regulators are calling for more detailed chemical analysis of drugs and the bourgeoning biologics market is driving competition. Meanwhile, a global conversation on drug prices means biopharmaceutical companies do need more cost-effective ways to develop new medications. Utilizing industry-leading skills and technologies to reduce the time and cost of bringing new products to market is the key to staying ahead of the curve.
Reference
1. https://www.globenewswire.com/news-release/2018/06/25/1529080/0/en/Biologics-Market-to-become-US-479-7-bn-by-2024-Says-TMR.html.
An Approach for Extractable & Leachable Evaluations of High-Risk Infusion Devices

James R. Scull, PhD, Division Director, Element Health Sciences has more than 32 years of pharmaceutical development experience spanning all areas from discovery support through manufacturing. His expertise includes applied analytical chemistry, toxicology, multi-dimensional separation science, extractable & leachable studies, CMC product development, and oligonucleotide analysis.
Over the past decade, regulatory agencies have placed specific interest in understanding the extractable and leachable (E&L) profile of container closure systems, manufacturing equipment, and medical device implants. The concerns of knowing such information are well-founded, as compounds leaching from these materials may impact the safety or efficacy of the drug or may have a direct impact on the patient in terms of an adverse reaction. While all types of infusion devices require an E&L evaluation, this paper will focus on the specialty subset of high-risk infusion devices and how to assess their unique attributes.
Study Rationale
Examples of High-Risk Infusion Devices are those used for intrathecal or intraperitoneal infusion. In these applications, the drug being delivered is intended to reside in the local infusion area for an extended period of time. Similarly, any leachable compounds from the infusion device would also do the same. These sites in the body have limited circulation. For example, the relatively limited circulation of spinal fluid potentially allows for a build-up of leachables in direct contact with the spinal cord. Similarly, limited circulation in the peritoneal cavity can allow for a pooling of leachables in direct contact with abdominal organs. In addition, the catheters and connection devices themselves may reside in the patient for weeks until treatment is complete.
For an infusion device, leachables may enter the body via two different routes: First, they may leach from the infusion set component into the flowing drug solution, which then carries the leachable into the body; or second, the leachable may diffuse from the component directly into the surrounding tissue. Therefore, both scenarios must be considered when designing an appropriate E&L study for such devices.
There are four critical parts of this E&L evaluation:
1. The extraction solvents selected must represent the anticipated use.
2. Analytical test methods must be validated for the target leachables identified.
3. The leachables assessment must be performed using clinically relevant conditions.
4. Manufacturers of these devices are required to demonstrate drug compatibility of the intended class of drugs to be infused with the infusion set, as a whole. The compatibility study must be done using a commercially available infusion drug that would be administered via the same route of exposure (i.e., intrathecal or intraperitoneal).
Evaluation of scenario two, in which the leachable migrates directly from the component into the body tissue, is simulated by exposure of the component in normal saline or simulated spinal fluid, in the case of an intrathecal device.
Component Selection & Test Methods
The components to be tested for extractables are those parts of the infusion device that have direct contact with either the drug or the patient. Ideally, each component should be tested individually. For components that cannot be separated from each other, the entire piece may be tested. The study design is a hybrid between ISO 10993-12 and the PQRI Guidance on Parenteral and Ophthalmic Drug Products. The ISO 10993 calls for exhaustive extraction. This can be determined gravimetrically or by instrumental techniques such as gas chromatography. The extraction is deemed exhaustive when the weight variance of the sample (gravimetric analysis) or the peak area of a specific compound (chromatography) is less than 10% between extraction cycles. Typically, 3 to 5 cycles are needed to achieve exhaustive extraction status.
The PQRI guidance plays a role in the study design by designating the instrumental techniques for analysis of the extract solutions. The extract solutions need to be tested for volatile and semi-volatile organics via HS-GC/MS and GC/MS, respectively. Additionally, these solutions need to be tested for non-volatile organics via HPLC/MS and for inorganics via ICP/MS. Reporting of the results should be in-line with the detection and quantitation limits of the individual techniques.
Summary
All of the details regarding this topic cannot be included in this brief article. However, it is obvious that high-risk infusion devices require special consideration when it comes to performing E&L studies, and the associated regulatory expectations are more rigorous than typical injectable products. Additionally, the concentration of each leachable compound must have a toxicological assessment performed to assess patient safety. But, that is a topic for another article.
The Analytical Method Life Cycle

Houri Simonian, PhD, has held the position of Director of Chemistry for SGS Mississauga for the past 5 years. She has more than 20 years of experience in the pharmaceutical industry, including R&D and drug development.
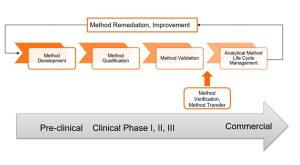
With the Product Life Cycle Management (PLCM) established as a fundamental part of the product development approach for pharma and biologics, attention has finally been drawn to Analytical Method Life Cycle Management (MLCM) as a key process during drug development.
The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and the United States Pharmacopoeia Forum (USP-PF) have initiated discussions and proposed drafts for the Analytical Life Cycle procedure. The QbD (Quality by Design) approach that has been applied to the manufacturing process for drug development has also been proposed for the Analytical MLCM. With a greater understanding during method development, establishing the analytical target profile, and challenging the method, with a risk-based approach and method controls, provides a broader knowledge base to ensure successful qualification/validation of the method and routine use.
The analytical procedure must be shown to be fit for its intended purpose before use. This must be considered with the critical quality attributes of the drug and the intended use of the method, such as identity, purity/impurities test, etc. Selecting the appropriate technique and gathering knowledge may depend on whether the method is new, adapted, improved, or an adoption of an existing procedure. Identifying the critical steps in the analytical procedure – the grade of reagents, standard and sample preparations, stability of the solutions, the recovery and precision of the analyte – can potentially influence the performance of the method. Establishing the sources of the variability or bias will lead to more rugged or robust procedures. Well-established, risk-based tools can be applied to identify the
variables and evaluate the impact to the procedures.
The result of these experiments typically leads to establishing the controls in the analytical methods. Determining the variables that need to be controlled and establishing acceptable criteria for these are captured in the analytical procedure. It is critical to take into consideration the type of technique and the intended use of the procedure when establishing the criteria. For example, limits on replicate preparations, calibration criteria, and system suitability requirements such as resolution and quantitation limits may be applied to chromatographic procedures, however the criteria for replicate preparations and additional controls may be applied for biological samples. Controlling the variables, with careful consideration of each parameter and criteria being monitored in the analytical procedure, is key to ensuring the reduced failure rate during routine use.
Having established the analytical target profile, with identified critical variables and measures in place, the method can be successfully qualified and/or validated. These next steps allow for additional opportunities to confirm and establish the critical attributes and validate them. Analysis of the qualification/validation data provides further confirmation for the analytical controls defined in the method.
After the validation of the analytical procedure, the final and continuous monitoring of the method is the significant step in the method life cycle management, as routine monitoring and trending provide direct evidence of the performance of the method and its fit for purpose. It is key to ensure that results are collected and analyzed with trending of the analytical data, system suitability failures, out-of-specification results, out-of-trend results, and stability trending. The evaluation of the data and trend may lead to further method development, for example, sample preparation variability. Emphasis should also be placed on the system suitability failures and any trends that, again, should lead to additional development and establishment of new criteria. Identifying the changes that are required and addressing these through the analytical method life cycle management ultimately results in more rugged and robust methods, faster routine analysis, and reduced out of specifications.
Applying the analytical method life cycle approach provides an enhanced approach to developing and validating analytical methods with an emphasis on the changes and continuous improvement throughout product life cycle.
Total Page Views: 6310