2023 Analytical Testing eBook – Analytical Testing Evolves With the Pharma Industry
By: Contributor Cindy H. Dubin
Outsourcing analytical testing continues to be a time and money saver for bio/pharma companies that do not have the necessary infrastructure to support these activities. Thus, the global pharmaceutical analytical testing outsourcing market was valued at $7.6 billion in 2022 and could reach $13.31 billion by 2028.1,2 The activities most outsourced are bioanalytical testing, method development and validation, and stability testing.1 And, based on product type, the active pharmaceutical ingredient segment leads the analytical testing outsourcing market, driven primarily by the rising demand in generic drugs.2
Alcami Corp.’s solid-state characterization is meant to advance a customer’s knowledge of their API, progress clinical candidates, and extend intellectual property. This service includes a myriad of tests, including polarized light microscopy, power X-ray diffraction, differential scanning calorimetry, crystallization screen, dynamic vapor sorption, and more. In the case of one client, Alcami performed solid-state characterization for a small-molecule API and is now progressing the program toward GMP manufacturing to support a Phase I clinical trial.
As the pharma industry evolves, so too is the analytical testing. The use of advanced analytics and artificial intelligence (AI) are becoming more widespread in pharmaceutical testing, helping to improve efficiency and accuracy. For example, AI-based image analysis can speed up identifying and characterizing particles in a drug substance.3
Stevanato Group’s Technology Excellence Centers, for example, are using computer simulation to predict the likelihood of rubber fragments contaminating medication during injection using a staked needle. Using the model to simulate real-world occurrences is streamlining product development and accelerating time to market, the company claims.
Like Stevanato Group, West Pharmaceutical Services helps pharma companies adhere to timelines by offering physical testing of container closure integrity testing for product packaging. According to the company, as packaging systems become more complex and new biologics are developed, the ability to store container systems in a comparable way to their lifecycle is of the utmost importance.
Learn more about Alcami, Stevanato Group, and West in this exclusive annual Analytical Testing e-book from Drug Development & Delivery.
References
1. Pharmaceutical Analytical Testing Outsourcing Market Size, Share & Trends Analysis Report By Service, 2023-2030, Grand View Research, April 15, 2023.
2. Global Pharmaceutical Analytical Testing Outsourcing Market Size, Share, Trends, COVID-19 Impact & Growth Analysis Report, 2023-2028, Market Data Forecast, March 2023.
3. Stay Ahead of the Game: The Latest Trends in Pharma Testing, By Brad Larmie, Jan. 20, 2023.

Case Study: Solid-State Characterization of Small Molecule API
 By: Joanna Bis, PhD, Associate Director, Solid-State Characterization at Alcami Corporation
By: Joanna Bis, PhD, Associate Director, Solid-State Characterization at Alcami Corporation
Client Profile
The client needed a routine hygroscopicity assessment to complete the solid-state characterization of their small-molecule API for inclusion in the IND application. The development of a formulation to support animal PK studies was already underway and involved the only known crystalline anhydrous form (ie, Form 1).
Partnering With Alcami
Alcami’s Solid-State Characterization team used gravimetric sorption analysis (DVS Intrinsic Plus – Surface Measurement Systems) to obtain the water sorption-desorption profile of Form 1. The DVS data indicated a complex solid-state nature of the API and likely a new hydrated form. The new form was stable above 50% relative humidity (RH), which overlaps with ambient humidities in laboratory and manufacturing sites. Powder X-ray diffraction (PXRD; Bruker D8 Advance) and hyphenated thermogravimetric analysis with IR detection (TGA-IR; TA Instruments 5500-ThermoFisher iS50) were immediately collected on the post-DVS solid and confirmed a formation of a new dihydrate (Form 2). Alcami collected and reported these data to the client within 72 hours, with a recommendation to incorporate drier conditions during relevant unit operations to prevent the formation of Form 2. Low-humidity conditions (<40%RH) were immediately applied during API handling, formulation, and packaging unit operations at Alcami and client sites.
In order to understand any risks to the formulation and crystallization processes of Form 1 that could be posed by the new Form 2 and other, potentially undiscovered solid forms, Alcami assessed the API’s polymorphic landscape. A polymorph screen was conducted and included 100 experiments, capturing diverse crystallization conditions (solvents, temperatures, etc.). Using an automated sample processing platform and high-throughput analytical tools, a new anhydrous form was identified (Form 3) within one week. Thermodynamic stability studies were conducted and showed that Form 3 was stable at elevated temperatures (enantiotropic form) that are utilized in the current cooling crystallization process of Form 1. Alcami’s team recommended the adjustment of seeding, isolation, and drying temperatures in the crystallization process to ensure a reliable production of phase-pure Form 1.
Alcami worked alongside the client to incorporate the changes to the ongoing formulation and crystallization process activities, supporting the quick progression of this program.
Challenges & Solutions
The program faced various challenges:
- Tight timelines imposed by the IND filing deadline were mitigated by flexible and efficient workflows leveraging the team’s deep experience and expertise;
- Extremely high solubility of API in water and organic solvents were mitigated by customization of solid and solvent dosing strategies and temperatures at the various stages of the polymorph screen;
- Interconversion of Form 1 and 2 at ambient humidity affecting analytical readouts were mitigated by use of a low humidity roomand proper testing schedules; and
- High potency of the API (1-10 μm/m³) requiring additional safety measures and sample containment during testing were mitigated by the existing safety-focused workflows.
Where Are They Now?
The work was completed on time and the content of the produced technical reports was directly utilized in the IND application, as well as in the patent application to secure the IP around the newly identified crystalline forms. Alcami is progressing the program toward the manufacture of GMP oral solid dose product to support a Phase I clinical trial.

How Computer Simulation is Shedding New Light on the Issue of Coring
 By: Alan Xu, Product Manager for Analytical Services at Stevanato Group
By: Alan Xu, Product Manager for Analytical Services at Stevanato Group
Staked needle syringes have many advantages – ranging from user convenience to a reduced risk of contamination. But the rubber closure that seals the needle comes with a potential risk of coring – when the needle shears out slivers of rubber as the two come together during assembly. So, what is the best way to minimize the risks to patients from tiny fragments of rubber contaminating their medication or even obstructing the injection process?
The rubber elastomer closure is often known as a needle shield – or rigid needle shield when a plastic cage surrounds the needle shield. It forms a sterile barrier to protect the syringe and any contents before use. Different rubber compounds, rubber designs, and needle geometries are selected by pharma companies or syringe manufacturers based on the type of drug product and how the syringe will be used. For example, staked needle syringes can then be prefilled and placed inside a drug delivery device like an autoinjector or pen injector. Or they can be empty, with the drug product aspirated from a larger container, such as a vial, into the syringe before injection into the patient.
The discovery of rubber fragments in some drug products ejected from such syringes has prompted investigations into whether there is always a sliver of rubber inside the needle when it is assembled with the needle shield or whether a core of rubber – a ‘coring phenomenon’ – is only formed under certain conditions. In the ideal scenario, the rubber would simply be compressed or displaced to one side, with no rubber inside the needle.
Stevanato Group’s Technology Excellence Centers (TECs) set out to discover if the likelihood of coring occurring could be predicted using computer simulations instead of empirically looking for rubber fragments using Design of Experiments (DoE) techniques each time a new tip cap and syringe combination is proposed. Two primary aspects were considered:
- The mechanical characteristics of the rubber with respect to the insertion/extraction motion
- The friction-based interaction between the rubber and the needle during extraction
The first step in developing the computer model was to conduct physical experiments on the rubber to characterize behavior that is not readily available in supplier datasheets. Once this material property is understood, the testing does not need to be repeated unless new material compounds are being analyzed.
A dedicated ‘simulation solver’ computer model was then created, taking into account the expected substantial deformation of the rubber and the cutting – or node separation – that may occur if rubber fragments are formed from the assembly of the needle and rubber or when the needle is extracted. Given that the cutting of rubber results in node separation, it was not feasible to model this using conventional numerical techniques. Consequently, an advanced meshless numerical technique was employed for this study, specifically designed for solid mechanics.
To see how well the computer simulation represented real-world observations, the simulation’s results were compared against those of a physical DoE. Twelve combinations were tested, based on the following variables: two needle types, two assembly speeds, and three rubber materials and/or designs.
In the worst case, the model directly predicts the formation of distinct pieces of rubber. These were strongly linked to specific rubber material types, but also affected by assembly speed (or the rate the rubber is strained). When comparing the numerical results of the simulations to the physical observations, high deformation values corresponded to the largest cores found, while medium deformation values corresponded to smaller, but still distinct cores.
Based on the strong correlation between the simulation and physical results, the Stevanato Group’s TEC model appears to be a reliable predictor of the real-world coring phenomenon. And one advantage of using such simulations is that, if further coring issues arise in the future, the same material, assembly, and needle parameters can be loaded into the simulation to save time on physical testing.
The simulation solver suggests that, in a root cause and failure recovery situation, changing the elastomer would be the most effective way to mitigate coring. If material changes are not possible due to existing stability or commercialization status, modifying the assembly parameters could be the next option to reduce the coring phenomenon.
Overall, Stevanato Group’s TEC coring model represents one of many successful digital twin models that can help streamline the product development and root cause investigation process to accelerate time to market and minimize downtime. Stevanato Group’s TEC is also developing other highly specialized and relevant simulation models for the pharmaceutical and combination product market.

Is it Ever Too Soon to Start Your Container Closure Integrity Assessment?
A Step-Wise Approach for Drug Companies to Meet Submission Timelines
 By: Jennifer Roark, Technology Manager, Container Closure Integrity, West Pharmaceutical Services, Inc.
By: Jennifer Roark, Technology Manager, Container Closure Integrity, West Pharmaceutical Services, Inc.
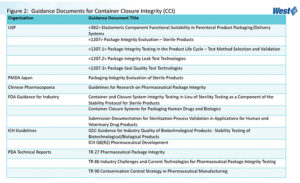
Demonstrating container closure integrity (CCI) for drug product packaging and delivery systems throughout the drug product lifecycle (package development, drug product manufacture, and commercial drug product stability) is a requirement for any regulatory submission. Agency expectations for CCI continue to evolve and expand through new regulations, such as EudraLex, The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use, Annex 1 Manufacture of Sterile Medicinal Products, which became final on August 22, 2022. Guidance documents, technical reports, and compendia also address the regulatory expectations and requirements for CCI that must be met throughout the drug product life cycle. These requirements are detailed in the United States Pharmacopeia (USP) general chapter <1207> Package Integrity Evaluation – Sterile Products and its sub-chapters, and in the new USP general chapter <382> Elastomeric Component Functional Suitability in Parenteral Product Packaging/Delivery Systems.
The expectations and requirements for CCI cannot be quickly addressed at the end of Phase III clinical trials. Rather, packaging requirements must be understood and CCI must be part of the Quality by Design (QbD) approach to drug product development early in the drug product life cycle. In addition, CCI must be monitored continuously throughout the shelf-life of the drug product. For many drug products, the typical shelf-life can range from two to five years. New companies in emerging markets often require assistance with CCI technology selection and face unique challenges with maintaining CCI for advanced therapies, which often require ultra-cold (-80°C) or cryogenic (≤ -130°C) storage and shipping conditions. If pharmaceutical companies wait until the end of Phase III to start planning their CCI study and testing program, they may face increased costs or delays in market launch due to incomplete submissions that may require the drug company to perform additional CCI testing. To meet regulatory expectations, the mindset around CCI testing must not be “How quickly can we get CCI testing done?” but rather “Is it too soon to start thinking about a CCI study?”
Why Should CCI Testing Start Early?
Treating CCI as a singular “test” would not satisfy regulatory expectations. On the contrary, CCI studies must be conducted during all three phases of the drug product life cycle beginning with package development, which means that CCI evaluations must start early in the drug product development process. CCI evaluations then continue through the drug product life cycle into the drug product manufacturing and commercial drug product stability phases. General information chapter USP <1207> and its subchapters, in conjunction with USP <382>, provide the guidance and regulatory expectations for CCI testing during these three phases. The process flow during each phase of the drug product life cycle is as follows:
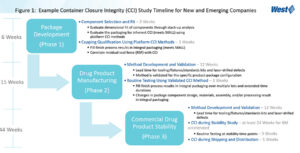
Phase 1 – Package Development
- Evaluate a variety of packaging component combinations for dimensional fit (i.e., stack-up analysis and/or or computational analysis such as the DeltaCube™ modeling platform); or evaluate a novel packaging system for potential leak paths. Platform methods for helium leak detection and residual seal force (RSF) analysis are ideal for this type of testing.
- Evaluate the packaging combination or drug delivery system for inherent CCI.
-The packaging meets the drug product requirements for maximum allowable leakage limit (MALL) and/or in-use MALL.
-The fill-finish process results in integral packaging. - Evaluate CCI during drug product shipping and distribution studies.
Phase 2 – Drug Product Manufacturing
- Products sealed under vacuum require fill-finish process validation and CCI testing to ensure that the vacuum and/or gas overlay is properly maintained.
- The fill-finish process continues to result in integral packaging over multiple lots and extended time durations.
- Changes in package component design, materials, assembly, and/or processing result in integral packaging.
-Use statistical sampling plans for CCI testing during lot release.
-Incorporate seal quality tests, such as RSF analysis, as described in USP <1207.3> as indicators of manufacturing process consistency.
Phase 3 – Commercial Product Stability
- CCI testing during a stability study evaluates whether package integrity is affected by the stability storage conditions.
- CCI testing is performed during drug product shelf-life studies in conjunction with sterility testing at lot release (T=0) and at the end of shelf-life.
- CCI testing determines if product-packages sealed under vacuum and/or with an inert gas overlay maintain the proper headspace gas over the shelf-life of the product.
To understand where CCI fits into the three phases of the drug product life cycle, and the amount of time required to execute each CCI activity, refer to Figure 1, which presents an overview of the CCI study requirements and approximate timelines for each phase. Different or multiple CCI technologies may be used during the phases of the drug product life cycle. Because the CCI method must be appropriate for the desired outcome and validated for the specific product-package, method development and validation can occur for more than one CCI technology during Phase 2 through Phase 3. For example, a helium leak method may be validated for use during Phase 2 drug product manufacturing; and a vacuum decay method may be validated for use during Phase 3 commercial drug product stability.
Drug Product Package Development
According to USP <1207.1>, package development is based on drug product-specific requirements, which include consideration of product end use, stability, manufacture, storage, shipment, and distribution. Component selection is based on critical physical attributes such as the materials of construction, the source of those materials, and dimensional fit. The way components are processed and assembled into a container closure system impacts package integrity. Therefore, it is important to understand during this phase if the package can achieve inherent CCI, which is defined in USP <1207> as “the leakage rate of a well-assembled container-closure system using no-defect package components.”
Inherent CCI provides assurance that the container closure system can meet the MALL for the drug product. USP <1207> defines the MALL as “the greatest leakage rate (or leak size) tolerable for a given product packaging/delivery system that poses no risk to product safety and has no impact, or inconsequential impact, on product quality.” The MALL must be determined for each specific drug product based upon the need to preserve sterility, product formulation content, and headspace gas content. During this phase, helium leak detection is often used to evaluate the inherent CCI of a container closure system. For vial-stopper-seal combinations, RSF analysis is performed in conjunction with helium leak detection to correlate RSF with CCI. To achieve CCI, the drug product manufacturing process must be in control. As a seal quality test, RSF can indicate manufacturing process consistency. Understanding the RSF value that correlates with inherent CCI determined using helium leak detection is a robust approach to understanding package integrity.
Drug Product Manufacturing
The seal quality and CCI technologies used during the package development phase can also be used during drug product manufacturing to ensure that the manufacturing process is consistent and in control. Other non-destructive CCI technologies such as vacuum decay or high-voltage leak detection (HVLD) can be used to evaluate package integrity during this phase. These technologies are not as sensitive as laser-based gas headspace analysis or helium leak detection but are acceptable to use once inherent CCI has been established. Often in-line laser-based gas headspace analysis, vacuum decay, or HVLD are used for 100% inspection during manufacturing. However, these technologies are also available in benchtop formats to support CCI testing during shelf-life studies. The CCI test method must be qualified or validated for use during this phase of the drug product life cycle, depending on the phase (1, 2, or 3) of clinical trials.
Due to supply chain issues, alternate packaging components, such as vials, stoppers, and seals may need to be substituted for the original product-package. In this case, inherent CCI must be confirmed using the alternate components. Changes in the individual components of a packaging/delivery system can significantly increase the amount of inherent CCI testing that would need to be performed. By this phase of the drug product life cycle, the CCI method has been validated, so the impact of any package component changes to the scope of the validated method must be considered, and supplemental validation of the CCI method may need to be performed.
Commercial Drug Product Stability
Sterility testing alone does not satisfy regulatory requirements for shelf-life studies. Therefore, CCI testing must be performed to supplement sterility testing throughout drug product expiry. As per USP <1207.1> Package Integrity Testing in the Product Life Cycle – Test Method Selection and Validation and the FDA Guidance for Industry Container and Closure System Integrity Testing in Lieu of Sterility Testing as a Component of the Stability Protocol for Sterile Products, sterility testing has both scientific and practical limitations which cause it to be a poor measure of product–package integrity (CCI) when performed as part of the stability program. In addition, sterility testing offers no assurance that a package, which may be in no danger of microbial ingress, is able to prevent product loss or is able to maintain the headspace gas environment necessary for product quality.
Common CCI technologies, both probabilistic and deterministic, are better at demonstrating the potential for product contamination during a stability study than sterility testing alone. At this phase of the drug product life cycle, the CCI method must be validated for the specific product-package to be considered appropriate for use. The combination of sterility testing at product release with CCI testing at each annual stability time point through expiration will meet current regulatory expectations for the evaluation of package integrity for your submission.
How Do We Get Started?
Before a CCI study can start, a sponsor must first communicate pertinent information regarding the container closure system and the drug product. This information includes the type of drug product (i.e., small or large molecule, solid powder, liquid, or lyophilized), type of container closure system (i.e., vial-stopper-seal combination, prefilled syringe, cartridge, IV bag, etc.), storage temperature, desired drug product shelf-life and shipping conditions/duration. The information gathered from the sponsor will aid in choosing the appropriate CCI technology and designing the CCI study. For example, West can recommend the CCI technology that is most compatible with the product-package and design a CCI study that achieves the desired outcome, such as leak path detection, leak path location, leakage rate measurement, liquid egress/ingress potential, or microbial ingress potential.
After this information is gathered and assessed, a CCI study can be designed using the applicable compendia and guidance documents detailed in Figure 2. CCI studies must be designed to appropriately demonstrate that the package preserves product contents, and that the product-package remains safe, effective, sterile, and free of contamination through expiry and use.
The execution of a CCI study typically involves the following steps:
- Assessment of the product-package and all relevant configurations (e.g., different concentrations of drug product, placebo, fill volumes, alternate container closure system components)
- Selection of appropriate CCI technology or technologies and study design
- Method development and validation
- Execution of the CCI study or routine testing
- Review and Reporting of results
CCI technologies are described in Figure 3.
Additional time for ancillary tasks related to the execution of a CCI study should be factored into your timeline. Some examples of these activities include:
- Vendor (external lab) approval, CDA and/or other agreements
- Study design with external lab
- Quote/PO process
- Fixture or tooling creation
- Laser-drilled defects creation for positive controls
- Protocol generation
- Sourcing, shipping materials
A method development and validation report that details the work performed and the results of the study will be issued after the study has been executed. The validated product-package specific test method that will be used for routine testing will also be provided. At the completion of a properly executed CCI study, a sponsor will have a thorough understanding of the container closure integrity for their product-package. The sponsor will have sufficient CCI data generated during the three phases of the drug product life cycle to meet regulatory requirements for their drug product submission.
Conclusion
A complete CCI assessment for a product-package is a significant process. Starting the assessment early during Phase 1 of the drug product life cycle is critical to meeting regulatory requirements and timelines for filing. Compressing the timeline can result in gaps, cause delays in product launch, and have undesirable financial implications. Therefore, it’s never too soon to start your CCI assessment!
If you would like to learn more about how West’s Analytical Services and Integrated Solutions can help with CCI testing, drug product packaging and delivery challenges, please visit us at http://www.westpharma.com/services.
We invite you to reach out Contact Us so that we can connect you with an account manager in your region.
West and the diamond logo and DeltaCube are trademarks or registered trademarks of West Pharmaceutical Services, Inc. in the United States and other jurisdictions.
Total Page Views: 3479