Issue:April 2013
ADVANCED DELIVERY DEVICES - IntelliCap: An Intelligent, Electronic Capsule for Oral Drug Delivery & Development
Characterization of drug absorption in vivo throughout the gastrointestinal (GI) tract is essential for successful oral modified-release product development. This can be challenging, costly, and time-consuming. Medimetrics has developed an electronic drug delivery and monitoring device, the IntelliCap system. By accurately controlling and targeting drug delivery along with simultaneous measurement of pH and transit, detailed in vivo data are captured quickly to effectively guide a modified-release development project. Future applications of electronic smart pill systems promise to open new perspectives for personalized therapies and disease management.
INTRODUCTION
The oral route of administration is by far the most preferred drug delivery route. Whenever possible, an oral dosage form is developed and deployed. Further, to improve patient convenience and compliance, once-daily administered formulations are preferred. For compounds with short half-lives, this has led to the development of extended- or modified-release products. The toolbox for oral modified-release development continues to be refined and improved but does suffer from several limitations. Development can be painstaking and prone to failure. There is a strong need for a quick and simple way to precisely and reliably control the delivery of the compound within the GI tract to determine whether a compound is a valid candidate for further modified-release development.
A technology force that exerts influence on almost every area is the use of electronics for control and for integration of data from multiple sources. This has long led researchers to envision creation of an electronic drug delivery pill. With the advancemen of electronic and mechanical technologies along with an impetus to bring new approaches into the world of drug development, the time is right for electronic oral drug delivery. A recent report from Market & Markets analyzed the Smart Pills technology market, in which a smart pill is defined as an ingestible capsule with miniaturized micro-electronics.1 The report cites a fast-emerging cross-platform technology market growing from $442 million in 2012 to an estimated $965 million by 2017. Within that market, the emergence and rapid growth in drug delivery and monitoring devices is anticipated.
Medimetrics is a pioneer in electronic oral drug delivery. The company has created and applies its IntelliCap® technology, the world’s first intelligent oral drug delivery and monitoring capsule. The IntelliCap system combines controlled drug release, patient monitoring, and real-time wireless communication. This combination of elements is applied to create a crucial tool for the development of oral modified- release products. The flexibilit and ease of use allows for rapid in vivo evaluation of a drug when delivered at precise rates and locations within the GI tract. Obtaining this information early in the development process saves time and money, and allows resources to be effectively allocated to candidates that show the greatest probability of success.3 Today the IntelliCap system is used as an effective tool within drug research and development. In the future, the advantages of electronic drug delivery will be exploited in therapeutic applications in which precise delivery, personalized behavior, patient monitoring, and integration into a connected healthcare system promises to enable innovative and effective therapeutic options to treat disease and improve outcomes.
THE INTELLICAP SYSTEM
Medimetrics has developed the IntelliCap system, a unique R&D tool for the targeted delivery of drugs within the GI tract. The IntelliCap technology provides a fast, cost-effective, and convenient means for the controlled release of drugs to specific sites in the GI tract. In addition, quantitative data such as GI residence times, temperature, and local pH are measured and recorded during th process. In the form of a capsule, the IntelliCap incorporates a microprocessor, battery, pH sensor, temperature sensor, RF wireless transceiver, fluid pump, and drug reservoir. A photograph of the capsule is shown in Figure 1. The photograph illustrates the construction of the capsule in two main subunits: the electronics body and the drug reservoir. With this modular design, the drug comes into contact only with the reservoir, which is made from inert polymer materials. To enable the capsule to release drug with flexible profiles over time, the drug payload is in the form of a liquid (solution, suspension, or gel).
The IntelliCap capsule communicates via a wireless transceiver to an external control unit worn by the test subject. IntelliCap technology features real-time wireless data recording, plus wireles remote control of dose delivery, giving researchers the ability to monitor the capsule’s progress through the GI tract and direct the delivery profile “on the fly.” The capsule measures pH and temperature nominally every 10 seconds and reports the data immediately for display on a control station computer.
Measurement of individual, local GI pH environment is essential to fully understand drug absorption and pharmacokinetics. First, the pH environment into which the drug formulation is released can have a strong influence on the solubility of the compound. Second, the p profile reveals location of the capsule within the GI tract. There is typically a sharp rise in pH as the capsule passes from the acidic environment of the stomach into the neutral environment of the duodenum. Subsequently, there is a fall in pH as the capsule passes from the small bowel into the colon. These landmarks are used for example by the SmartPill system to measure and diagnose motility disorders.4,5 In an IntelliCap study, these landmarks are used to determine location of the capsule in order to control delivery to a targeted region and later on, to determine individual GI transit times to calculate local drug absorption in a pharmacokinetic (PK) study.6
APPLICATIONS IN DRUG DEVELOPMENT
Development of oral controlled-release products is often challenging, prone to errors and delays. Modeling or preclinical testing does not fully predict behavior in vivo. To accurately understand the in vivo properties of a compound and rationalize its development efficiently, the absorption of the product throughout the entire GI tract must be well characterized. A traditional way to determine this is to develop one or several extended-release formulations and test them in the clinic. This takes time and resources, eventually jeopardizing clinical development timelines and marketing application. In early stages of drug development, the question is often whether the compound is a good candidate for a modified-release formulation and thus worth the investment for such a development route. This creates a “chicken or egg” problem. In addition, modified-release technology is tuned toward the time constants and physiological properties of the human GI tract and therefore cannot easily be applied to preclinical models like dog for example.
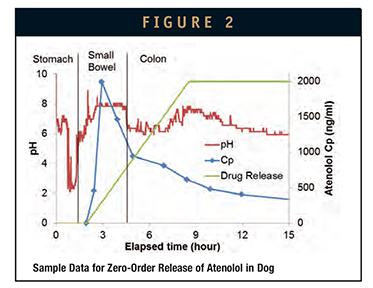
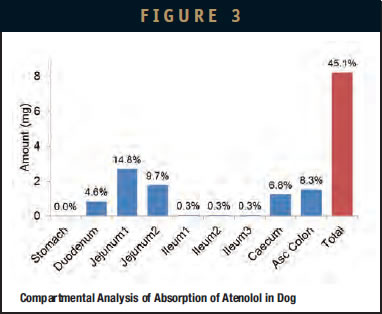
The IntelliCap system can be used to quickly design and complete a study in either a preclinical (animal model) or clinica setting. The drug-release profile is fully programmable and may be adapted to the individual GI transit properties in different species. The following example illustrates such a scenario. A study was performed in five beagle dogs with a reference arm in which a known compound, atenolol, is targeted for release into the small bowel and colon.7 Start of release is manually triggered after the capsule passes into the small bowel. The release profile chosen is a zero-order linear release over 6.5 hours ensuring compound delivery both into the small bowel as well as into the colon. The average transit in the small bowel of a beagle dog is about 2 hours. An example of the captured pH and PK data for a representative subject is shown in Figure 2. The pH data provides information as to location of the capsule in the stomach, small bowel, and colon. These individual transit times are used during the analysis of the PK data. Results for the compartmental absorption while accounting for individual transit with modeling software (GastroPlus, Simulations Plus) are shown in Figure 3. This example shows moderate colonic absorption, consistent with published results. Notice how absorption throughout the entire GI tract was studied with a single administration. This strategy allows for rapid assessment and characterization of local compound absorption properties that are hardly achievable by other means. This principle can be as well applied in clinical development. Adding an IntelliCap arm in a first-in-man study provides an early proof-of-concept for modified-release feasibility and initial data for further clinical development.
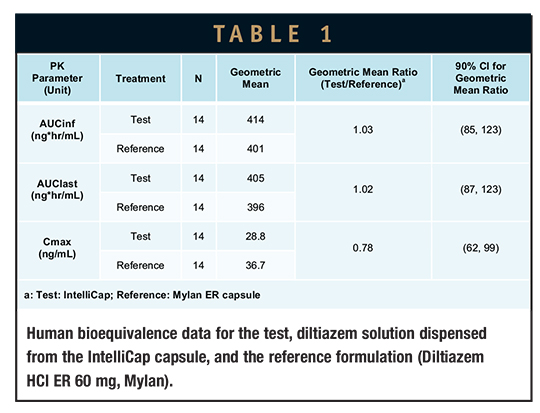
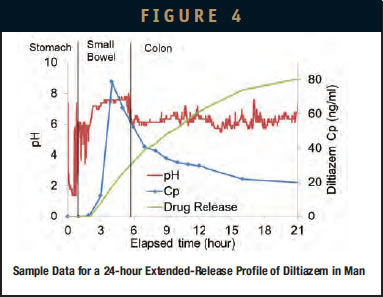
As a drug candidate advances toward modified-release formulation development, it is critically important to quickly evaluate how the drug-release profile translates into in vivo PK properties. Typically, one or more formulations are created and then tested in a clinical study. If results are unsatisfactory, the process must be repeated or the project is abandoned. The programmable nature of the IntelliCap capsule allows the release profile to be explored, altered, and adapted quickly to determine up-front (ie, before committing resources for solid dosage form development) the optimal release profile. This rapid formulation prototyping approach is illustrated in the following example. A first-order, 24-hour drug-release profile is programmed in the IntelliCap capsule mimicking the in vitro dissolution profile of a commercial extended-release product (Diltiazem HCl ER 60 mg, Mylan). The programmable nature of the IntelliCap system allowed the profile to be reproduced and verified in vitro within days. The reference commercial extended-release formulation and IntelliCap were then clinically evaluated in a small number of healthy volunteers (pilot bioequivalence study).8 Bioequivalence data between the test and reference formulations are shown in Table 1. Sample data from a representative subject for IntelliCap delivery is shown in Figure 4. The study illustrates how an arbitrary release profile can be quickly generated and tested in man and how the IntelliCap system allows for the rapid evaluation of the optimal release profile before committing time and resources to formulation development.
FUTURE APPLICATIONS FOR ELECTRONIC DRUG DELIVERY
As electronics and wireless devices continue their relentless push into ever more areas of our lives, so too shall we see the emergence and growth of smart electronic pills. A smart drug delivery pill promises to bring unique capabilities to treat disease and manage care. The combination of drug delivery, monitoring, and communication will enable a range of applications bringing oral drug therapies from uncontrolled bioactive deposition to accurate sitespecific personalized drug delivery and disease management.
Targeted topical drug delivery has many potential advantages for the treatment of locally active disease of the gut, such as inflammatory bowel disease (IBD), intestinal cancers, and irritable bowel syndrome (IBS). Topical delivery only to the region of involvement may reduce toxicity from systemic exposure and limit the formation of antibodies for biologics therapies. Region of involvement can vary from patient to patient and within a patient over time. The programmable nature of an electronic pill allows the target site and dose to be personalized. Localized and controlled delivery has applications beyond diseases of the gut. Delivery of peptides or other large molecules may be combined with delivery enhancers and location targeting to achieve effective oral delivery. Oral vaccines for example may be protected from the degrading environment of the upper GI tract and delivered to the ileum, where they are presented to antigen sampling cells.
In addition to site-specific drug delivery, the electronic pill may also incorporate biomarker sensors and reporting of measurement and actions from within the gut. Sensor measurements may be transmitted wirelessly, and data integrated automatically into the patient’s health record for reporting, diagnostics, and management of long-term treatment. The microbiota environment and balance of the gut is increasingly recognized as critical to overall health.9 Monitoring, managing, and treating gut health may become a key part to healthy living, and the electronic pill can play an important role.
CONCLUSION
Electronic smart pills represent an emerging area with great potential for growth. Already established in the diagnostic area, the next move is into monitoring and drug delivery. Drug delivery from a swallowed electronic device brings several advantages an opportunities. Medimetrics is a pioneering entrant into this segment, and its IntelliCap system is an approved measuring and drug delivery device available today. The first application is in drug delivery studies in which controlled targeted delivery along with measurements of individual transit and pH enable quick and accurate data of in vivo properties for a drug in development. This is particularly valuable for modified-release development and for compounds in which local enteric delivery is central to the product target profile. Looking forward, the electronic smart drug delivery pill enables a range of novel therapies and transforms the conventional, swallowed pill to a key building block of a future personalized and interconnected healthcare environment.
REFERENCES
1. Smart Pills Technologies Market (2012-2017) (Diagnostic Imaging, Patient Monitoring, Drug Delivery). Markets & Markets. 2012;PH1303.
2. Shimizu J, et al. ePill – an electronically controlled oral drug delivery platform. AAPS Journal Suppl. 2008;10(S2):1696.
3. Thombre AG. Assessment of the feasibility of oral controlled release in an exploratory development setting. Drug Discov Today. 2005;10(17):1159-1166.
4. Rao SS, et al. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7(5):537-544.
5. Kuo B. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27(2):186-196.
6. Zou H, et al. Regional drug absorption study in canines with intelligent pill system. Paper presented at: 37th Annual Meeting Controlled Release Society. 2010;5373-1.
7. Becker D. IntelliCap – a new device – paves the way for a guaranteed successful modified release development. Paper presented at: 2nd Annual Innovations in Modified Release. 2012.
8. Becker D, et al. Quantitative determination of regional drug absorption in dog and man with IntelliCap system towards optimized success in MR development. AAPS Journal Suppl. 2012;14(S2):W4055.
9. The Gut Microbiota, Special Issue. Science. 2012;336(6086).

Jeff Shimizu is a pioneer of the IntelliCap drug delivery system, Cofounder and CTO of Medimetrics. He has a background in physics and optics. Prior to Medimetrics, Mr. Shimizu had been with Philips Research for over 20 years in systems research and development. Research into medical devices led to the development of IntelliCap, an electronic drug delivery capsule. Medimetrics was formed to bring this technology to the market.

Dr. Christoph Wanke is Clinical Program Leader at Medimetrics. With over 15 years of experience in pharmaceutical industry, he is responsible for the preclinical and clinical development of the IntelliCap system and customer services. Dr. Wanke earned his PhD from the Swiss Federal Institute of Technology in Zurich.
Total Page Views: 12134