2022 Analytical Testing eBook – Service Providers Offer Innovation
Competitive pressure, rising costs, and time to market all play into the decision to outsource analytical testing of new drug product. This is leading industry gurus to value the global pharmaceutical analytical testing market at $12 billion by 2027.1
There is more demand for particular kinds of tests as a result of the development of biosimilars, combination products, and other novel medicines. The bioanalytical testing segment is expected to soar due to rising R&D spending by players in the biopharmaceutical industry and a preference for outsourcing analytical testing. The segment for bioanalytical testing has expanded as a result of the rising number of clinical trial registrations and the entry of new players into the market over the past decades.2
A variety of testing services are offered by providers. The extractable and leachable services segment is expected to witness aggressive competition, owing to an increase in the number of vendors offering these services at competitive prices. These studies are conducted to comprehend the potential for impurities in formulation to escape after packing.
Stevanato Group, for example, helps with method development to detect glue-derivative compounds used in staked needle syringes that may interact with the drug product. If not detected, loss of drug potency is possible. And West Analytical Services performs simulation and migration studies, risk assessment, method development and validation, stability testing, and toxicology support.
It is also anticipated that the current demand for therapeutics and vaccines against COVID-19 will increase demand for sustained capabilities in many laboratories to perform bioanalytical assays. Alcami uses Inductively Coupled Plasma Mass Spectrometry (ICP-MS) to assay metals that are intentionally added to pharmaceuticals as a major component of the formulation. The company claims that ICP-MS has proven to successfully develop, validate, and transfer assay methods precisely and accurately.
Testing for endotoxins remains a critical in-process and final release test for parenteral products. And, different approaches have been developed to provide solutions for the breadth of product range that is tested for endotoxins, such as Limulus Amebocyte Lysate (LAL). Associates of Cape Cod’s Pyrosmart NextGen® is a sustainable recombinant LAL Cascade reagent (rCR) that is horseshoe crab blood-free for Bacterial Endotoxin Testing (BET).
Learn more about the technologies from Alcami, Associates of Cape Cod, Stevanato Group, and West in this exclusive 2022 Drug Development & Delivery Analytical Testing e-book.
References
1. Pharmaceutical Analytical Testing Market – Growth, Trends, COVID-19 Impact, and Forecasts (2022-2027), ReportLinker, April 22, 2022, https://www.globenewswire.com/news-release/2022/04/22/2427170/0/en/Pharmaceutical-Analytical-Testing-Market-Growth-Trends-COVID-19-Impact-and-Forecasts-2022-2027.html.
2. U.S. Pharmaceutical Analytical Testing Outsourcing Market Size, Share & Trends Analysis Report By Service (Bioanalytical Testing, Method Development & Validation, Stability Testing), By End-use, And Segment Forecasts, 2022-2030, Grand View Research, July 18, 2022, https://www.grandviewresearch.com/industry-analysis/us-pharmaceutical-analytical-testing-outsourcing-market.
 Sustainable Recombinant LAL is Looking Bright
Sustainable Recombinant LAL is Looking Bright
By: Veronika Wills, Manager, Technical Services, Associates of Cape Cod, Inc.
The horseshoe crab has played a crucial role in human health. The animals’ bright blue blood contains Limulus Amebocyte Lysate (LAL), a substance used for bacterial endotoxin testing. LAL clots when it encounters bacterial endotoxins, enabling the detection of the potentially deadly contaminants in parenteral drugs, vaccines, and medical devices. Conservationists claim that hundreds of thousands of horseshoe crabs are captured and bled annually. These groups argue that alternatives exist to render this practice unjustifiable.
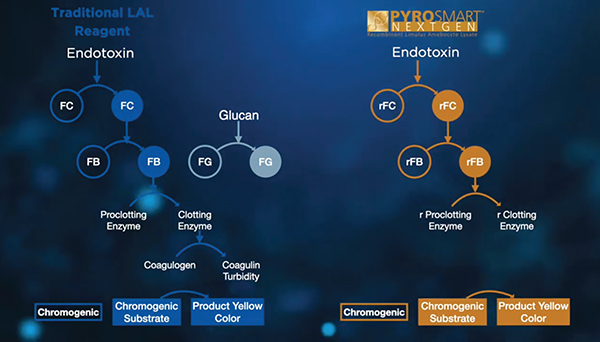
After years of research and development, Associates of Cape Cod (ACC) has been able to bring a truly sustainable alternative LAL reagent, designed to deliver consistent and reliable quantitation in all BET applications requiring LAL reagents such as testing of water, injectable drugs, vaccines, and medical devices. ACC’s PyroSmart NextGen® is a recombinant Cascade reagent (rCR) that is horseshoe crab blood free. PyroSmart NextGen® is a sustainable recombinant LAL reagent technology for Bacterial Endotoxin Testing (BET). Utilizing the same LAL cascade as traditional LAL reagents, and with the added advantage of eliminating the potential for 1,3-β-D-glucans cross reactivity, PyroSmart NextGen® provides the same result consistency, without any reduction in quality.
PyroSmart NextGen® can be used for a wide variety of endotoxin tests, ranging from standard water testing to samples requiring high sensitivity, such as intrathecal products and those requiring high dilutions to overcome interference. The sensitivity for recombinant chromogenic assays is determined by the lowest standard concentration on the standard curve used for the assay. The maximum sensitivity of PyroSmart NextGen® is 0.005 EU/mL when run in an incubating microplate reader (or 0.001 EU/mL when run in Pyros Kinetix® Flex tube reader).
PyroSmart NextGen® is used with an economical volume of 50 μL of reagent per well yielding 50 tests per vial. The PyroSmart NextGen® reaction mixture is incubated at 37±1°C and read in a microplate reader equipped with a 405-410 nm filter. The time of incubation is dependent on the lowest standard concentration in the standard curve, with 0.005 EU/mL achievable in 2,500 seconds in a microplate reader. Software is used to construct the standard curve and calculate the endotoxin concentrations.
The reagent is provided as co-lyophilized with the chromogenic substrate and as such it is ready-to-use following a simple reconstitution (using 2.8mL of the supplied reconstitution buffer). The lyophilized product has an excellent shelf life of three years from the date of manufacture.
Switching to this sustainable alternative is easy because PyroSmart NextGen® follows the same cascade pathway as traditional reagents. And you can keep your method, no change to instruments or preparation steps. ACC developed PyroSmart NextGen® to provide a sustainable alternative to traditional naturally sourced LAL reagents, while maintaining your lab procedures, methods, instrumentation and, most importantly, your results.
ICP-MS: Leveraging Inductively Coupled Plasma Mass Spec to its Full Potential
The analysis of metals in pharmaceutical actives, raw material, and drug products has changed. ICP-MS/OES has emerged as a critical analytical platform for this analysis. Multiple ICP-MS capabilities to support this need, including catalyst control, assay determination, organometallic compounds, and cleaning verification methods, in addition to elemental impurities testing (raw material and product testing), serve to maintain regulatory compliance and help deliver safe products to consumers.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is a quickly growing technique in the pharmaceutical industry for its ability to deliver accurate and precise measurements of many metals within minutes. The general flow of analysis for ICP-MS starts with sample preparation, which can be as simple as diluting the sample material into a dilute acidic matrix or more complex preparation schemes that utilize extraction or digestion techniques. After the sample is in solution, it is aspirated into a fine, aerosolized mist that is introduced into the argon plasma. The argon plasma ionizes the atoms which then are streamlined towards the detector with the help of ion-focusing cones and lenses. Multiple configurations, including collision and reaction cell gases, can be interchanged to optimize the instrument’s ability to detect the analyte in both simple and complex sample matrices. The versatility, short analysis time, and ability to measure many elements at once make ICP-MS a better alternative to the older standby methods of flame and graphite furnace atomic absorption.
ICP-MS Applications
One of the most prominent sources of metal contamination derives from active pharmaceutical ingredients and excipients. In some cases, this is because metal catalysts like Ni, Pd, Pt, Cu, and Rh are required for the synthesis of these molecules. This necessitates controlling the number of catalysts that remain in the material at the completion of the synthesis. Alcami has developed and validated methods for monitoring metal catalysts (such as Pd and Pt) in raw materials to ensure that residual amounts of catalyst are under control. The limits applied in these applications is often far below the ICH Q3D elemental impurities option 1 limit. Alcami can develop and validate an ICP-MS method to analyze ppb level limits of metal catalysts.
ICP-MS is also valuable for assaying metals that are intentionally added to pharmaceuticals, such as platinum-based cancer medicines, where the metal of interest is the major component of the formulation. At Alcami, we have demonstrated the ability of ICP-MS to achieve precision and accuracy required for assay analysis by transferring, developing, and validating methods for assay. Alcami has transferred ICP-OES assay methods to ICP-MS, for sodium, with accuracy and precision better than 2%. We have also developed and validated for magnesium assay in various magnesium sulfate intravenous (IV) solutions, with similar accuracy and precision (2%). In addition to single-element assays, the method development and validation of an assay for multiple metals (Cr, Mg, Zn, Se, and Cu) simultaneously in various nutritive IV formulations demonstrated the ruggedness of the ICP-MS and the ability to perform fast, accurate assay in the presence of very complex sample matrices. Interference control by the ICP-MS instrument and the use of an internal standard allowed us to perform precise and accurate determination assay on the elements of interest.
Oraganometallic compounds are also becoming more common in the pharmaceutical industry. These compounds allow enhanced and/or novel functionality of therapeutic compounds in patients. Because organometallic compounds contain bound metals in their structure, the analysis presents additional challenges. Use of organometallic compounds requires the assay for intact organometallic molecule, as well as measurement of the residual unbound metal. This testing extends beyond the API because analysis of the unbound metal is required to show the molecule maintains its structure through drug product manufacturing and shelf life.
The low detection limits and quick turnaround times that ICP-MS offers has advantages for verification of pharmaceutical manufacturing equipment cleaning processes. Several cleaning verification methods have been developed by Alcami for releasing manufacturing equipment. If the API or drug product contains a consistent and known concentration of an ICP-MS-compatible element, a cleaning verification method via ICP-MS is possible and can be more sensitive than TOC and HPLC methods. By determining the maximum allowable carry-over (MAC) for the equipment and converting to the weight percent of the element of interest, the ICP-MS is sensitive enough to reach these levels. One of the methods developed by Alcami was able to determine tin with a method quantitation limit of 30ppb, with sample stability of four days, allowing manufacturing plenty of time to sample the equipment and send for analysis. Another cleaning method for gallium was developed, due to the presence of gallium in the active, with a method quantitation limit of 100ppb. Using ICP-MS for cleaning verification methods also allows for variable sample matrices, unlike TOC. The time for analysis can be as little as a few minutes per sample, which allows manufacturing equipment to be released for use in a timely manner.
ICP-MS can be used not only for elemental impurity testing pharmaceutical applications but has the potential to meet the changing needs of our industry.
 The Seemingly Innocuous Glue in Staked Needle Syringes
The Seemingly Innocuous Glue in Staked Needle Syringes
By: Alan Xu, Product Manager for Analytical Services at Stevanato Group’s Technical Excellence Centers, USA and Italy
Guidelines for container closures cover a variety of elements including particle counts and plunger glide force. But the adhesive used in staked needle syringes is an under-characterized realm. While glue or adhesive suppliers may provide preliminary extractable data, the final gluing and curing process parameters may include – and generate – new compounds that affect the final extractables profile. The true effects, such as loss of drug potency, may not be seen until the drug is in direct contact with the final container. If your drug is losing potency – or you wish to evaluate glue compatibility earlier to prevent late-stage formulation changes – where do you start?
Stevanato Group’s Technology Excellence Centers (TEC) can provide a helping hand with the Design of Experiments (DoE) and the associated method development to detect specific glue-derivative compounds that interact with your drug product. SG TEC can also help support method validation and transfer methods to your lot release provider to ensure future lots meet this new quality specification. With locations in Boston and Piombino Dese, Italy, SG TEC has extensive experience in a variety of techniques ranging from chemical investigations, such as adhesive compatibility and tungsten compatibility, to mechanical tests such as container closure integrity testing and break-loose and glide-force testing.
If you’re finding an incompatibility between your drug and an existing container, our investigation starts with a fish bone chart (or Ishikawa diagram) to evaluate all aspects of the process – from materials, machines, and the environment to human aspects, methods, and measurement. Next comes risk assessment to evaluate likely and unlikely items from the fish bone chart.
A series of experiments then needs to be designed to confirm the potential root causes. Experiments may include intentionally recreating the issue and testing high-impact process parameters at relatively high and low values. Fringe cases may also need to be evaluated – for example, perhaps the product is affected when the production line is on hold due to sudden maintenance.
For the most robust study, these DoE samples can also be included in a stability study to evaluate the long-term performance and interaction between these optimized parameters and the actual drug product. Based on the test results, an inspection criteria and/or process optimization that minimizes drug interaction can be finalized.
With acrylic-based glues, SG TEC has investigated and developed multiple techniques, including High-Performance Liquid Chromatography (HPLC) and Ion Chromatography (IC) test methods capable of quantifying the presence of acrylic acid and other glue compounds in the final cured adhesive. With our HPLC method, even individual syringes can be evaluated without having to pool multiple samples. Inspecting individual syringes allows a closer look at a process because there may be significant differences – even between pieces of the same batch.
Other crucial elements include specificity/selectivity; accuracy and precision, i.e. the closeness of the results to a true value and to results obtained by the same or different preparations; repeatability to show capability across multiple set-ups; linearity – whether the method correctly detects concentrations ranging from small to large; and robustness, with consistent performance under different variables. Once the method is validated, a method transfer needs to be performed to implement the method at the customer’s quality control laboratory, if they would like to implement it as part of the lot release process. Another fish bone and risk assessment is performed to plan the smoothest transition from one laboratory to another. And a failure modes and effects analysis (FMEA) evaluates the entire analytical process – from the infrastructure of the laboratory to the availability of the reagents/materials used in the method and the data analysis/reporting technique.
The attributes from the method validation are re-evaluated and compared between the two laboratories to see if equivalent results are obtained when testing the same material. In-person training is provided, where possible, and videos or live webcam training is provided if travel is not possible. Support for the generation of standard operating procedures at the receiving laboratory is also provided. Future planning may include a well-defined design verification testing plan to avoid problems further down the line.
With the right DoE, method development, and method transfer, container compatibility issues don’t need to derail your successful commercial launch of a world-class drug delivery device.
 Understanding the Regulatory Guidance for Extractables & Leachables That Impact Drug Combination Product Submissions
Understanding the Regulatory Guidance for Extractables & Leachables That Impact Drug Combination Product Submissions
By:Jennifer Riter, Senior Director, Business and Technical Operations, Services & Solutions; and Matt Woods, Manager, Extractables and Leachables, Analytical Services, West
![]()
 As regulations continue to evolve, and development of new combination products are increasing, it is important to understand the regulatory expectations and the data that is needed for the regulatory application. Submission of combination products is going to depend on the primary mode of action (PMoA), which determines the regulatory pathway of the combination product.
As regulations continue to evolve, and development of new combination products are increasing, it is important to understand the regulatory expectations and the data that is needed for the regulatory application. Submission of combination products is going to depend on the primary mode of action (PMoA), which determines the regulatory pathway of the combination product.
The regulatory agencies for submissions are: CDER, CDRH or CBRE. Looking at the drug development side, there are different types of guidance, such as the FDA Container Closure Guidance: Container Closure Systems for Packaging Human Drugs and Biologics, May 1999, ICH Q8 and Q9 for Quality by Design (QbD), as well as the CDRH biocompatibility such as a USP <87>:Biological Reactivity Tests, In Vitro, USP <88>: Biological Reactivity Tests, In Vivo, and USP <1031>: The Biocompatibility of Materials Used in Drug Containers, Medical Devices and Implants.
On the device end, there are the Medical Device Regulation (MDR) and General Safety and Performance Requirements (GSPRs) in the EU, as well as the ISO 10993 Biological Evaluation of Medical Devices series. Understanding how these need to be brought together for combination products is critical to the development strategy. Therefore, we have to bring together the drug and the device to assure that we are paralleling the development of these areas together as a combination product.
Types of Combination Products
• Single-entity, such as a prefilled syringe system
• Co-packaged products, such as a vial adapter that would be co-packaged with the drug product
• Co-labeled product where a specific drug product is labeled for use with another specified drug device or biologic
It is important to consider both the drug and device cGMPs, including determining the extractables and leachables approach for both the primary containment system and the device. It is important to understand the materials characterization, which includes the extractables and the biocompatibility. It is also important to understand the compatibility of the drug product, the containment system, and constituents.
Executing a Successful Extractables Assessment
The challenge in evaluating the safety and efficacy of materials used in combination products is that the combination product serves the role as both containment (packaging) and delivery (device). However, the documents available (ISO-10993 part 181 and USP <16632>, <16643>) to guide the evaluation of these materials are more specific to an action, either containment or delivery. There are technical differences in how to execute studies in these documents due to their focus on a specific action. These technical differences can be challenging for those unfamiliar in designing and executing extractable and leachable assessments.
The general blueprint to evaluate materials is consistent between the two guidance documents:
• Make good decisions on component selection.
• Understand what compounds could migrate from the materials and interact with the patient or drug products.
• Assess the risks associated with those compounds; how they pose a risk to the patient or product.
• Determine any appropriate final steps to be able to demonstrate the safety and efficacy of the materials within the combination product.
Steps for Performing an Extractables Assessment
If the sponsor focuses on making justifiable decisions using strong scientific rationale while traversing the extractables and leachables journey, they will have a successful technical execution of the assessment. Consider the following steps for performing a successful assessment.
1. Knowledge of Product: Information must be gathered on the composition and/or potential extractables of the material to make an educated decision on component selection. Using well-characterized materials sourced from reputable vendors mitigates the risk when selecting materials for a container closure system and device.
2. Evaluate Available Extractables Data: The data gathered should be evaluated against the appropriate guidance documents. The materials used to generate the data must represent or bracket the specific material being used. Additionally, the conditions of the study must adequately stress materials both based on the intended use of the materials and in compliance with regulatory expectations. Any gaps identified must be addressed with appropriate complementary extractable studies.
3. Design Extractables Study to Address Gaps in Knowledge: Design and execute an extractables assessment based on the intended use of the material consulting the appropriate guidance document. Risk can be further mitigated during the extractables assessment by consulting the ultimate decision makers from the start. Ensure that the agency and toxicologist agree that the study design will provide the information to support the safety and efficacy of the material.
After completing an extractables, or chemical characterization assessment, assess the data generated. A sponsor or manufacturer must assess the risk associated with the compounds generated under worse-case conditions to understand the risk those compounds pose to the efficacy of the drug and the safety of the patient. Additional studies may be necessary to support this assessment and aid decisions moving forward. These additional studies may include:
• Compound Identifications – Identify (or confirming identifications) of compounds reported in the extractable assessment.
• Simulated Leachable Screening Studies – Understand what can leach out of these materials using actual use conditions.
• Toxicology Assessments – Determine the compounds of risk, and at what levels they are a risk, to the patient.
A sponsor will use all of the data collected to guide the leachables study design. Specifically, the sponsor must determine what compounds present a risk to the patient or the efficacy of the drug product to target during the leachable study. Methods must be developed and validated targeting these compounds at the appropriate levels. The validated methods are then used to monitor the packaged drug product though out the shelf life. It’s also recommended to continue to use screening methods to supplement the validated methods throughout the leachable study to further support the decisions made on targeted compounds.
References
1. Biological evaluation of medical devices – Part 18: Chemical characterization of medical device materials within a risk management process.
2. Assessment of extractables associated with pharmaceutical packaging/delivery systems.
3. Assessment of drug product leachables associated with pharmaceutical packaging/delivery systems.
Total Page Views: 4683


















