Theratechnologies Announces New Findings for its Lead Investigational Compound for the Treatment of Several Additional Cancers
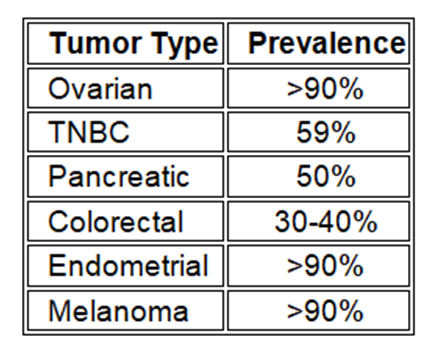
Theratechnologies Inc. recently announced new preclinical in vivo findings regarding the efficacy and tolerability of its novel investigational proprietary peptide-drug conjugate (PDC), TH1902, for the treatment of several cancer types expressing the sortilin receptor (SORT1+) as shown in the table below.
New preclinical in vivo results in colorectal, pancreatic, melanoma, and endometrial cancers are similar to those presented at the American Association for Cancer Research last June, which confirmed, at the time, the effect of TH1902 in vivo in ovarian and triple-negative breast cancers (TNBC). The company intends to present the detailed results at scientific meetings next year.
In addition, based on recently completed IND-enabling toxicity studies, Theratechnologies confirmed that TH1902 could be administered at three times the maximum tolerated dose (MTD) of docetaxel alone.
“These new results are very encouraging for the development of TH1902 in SORT1+ cancers and confirm that TH1902 can be effective in several different cancers expressing sortilin receptor. These data also support that TH1902 has the potential to have an improved safety profile at therapeutic doses in comparison to standard cytotoxics. Most importantly, these findings give hope that we may finally be able to tackle hard-to-treat cancers with a more effective and better-tolerated treatment,” said Dr. Christian Marsolais, Senior Vice President and Chief Medical Officer, Theratechnologies.
Prevalence of Sortilin expression by cancer type
Cancers expressing SORT1 (known approximate percentage by cancer type)
TH1902 combines Theratechnologies’ proprietary peptide to docetaxel. This peptide-drug conjugate (PDC) is the lead candidate stemming from Theratechnologies’ SORT1+ Technology in oncology. It is currently being studied for the treatment of cancers where the sortilin receptor is expressed.
The Canadian Cancer Society and the Government of Quebec, through the Consortium Québécois sur la découverte du médicament (CQDM), will contribute a total of 1.4 million dollars towards some of the research currently being conducted for the development of Theratechnologies’ targeted oncology platform.
TH1902 combines Theratechnologies’ proprietary peptide to docetaxel. This peptide-drug conjugate (PDC) is the lead candidate stemming from Theratechnologies’ SORT1+ Technology in oncology. It is currently being studied for the treatment of cancers where the sortilin receptor is expressed.
Theratechnologies has developed a peptide which specifically targets Sortilin (SORT1) receptors. SORT1 is expressed in ovarian, triple-negative breast, skin, lung, colorectal and pancreatic cancers, among others. SORT1 plays a significant role in protein internalization, sorting and trafficking, making it an attractive target for drug development.
Commercially available anticancer drugs, like docetaxel, doxorubicin or tyrosine kinase inhibitors are conjugated to Theratechnologies’ investigational novel peptide to specifically target Sortilin receptors. This could potentially improve the efficacy and safety of those agents.
Theratechnologies is a biopharmaceutical company focused on the development and commercialization of innovative therapies addressing unmet medical needs. For more information, visit www.theratech.com.
Total Page Views: 1700









