Monopar Announces Positive Early Human Clinical Data Validating the Tumor Targeting Ability of MNPR-101-Zr
Monopar Therapeutics Inc. recently announced positive early data from its ongoing open-label MNPR-101-Zr Phase 1 imaging and dosimetry clinical trial confirming MNPR-101-Zr’s tumor targeting ability in humans.
MNPR-101 is Monopar’s proprietary first-in-class humanized monoclonal antibody that targets cancers expressing the urokinase plasminogen activator receptor (uPAR). These include a majority of all triple-negative breast, colorectal, bladder, ovarian, gastric, and pancreatic cancers.
A total-body positron emission tomography (PET) image was taken at 168 hours (7 days) post administration of MNPR-101-Zr (a zirconium-89 imaging radioisotope conjugated to MNPR-101) of the first cancer patient in the trial with one of the known high uPAR-expressing cancer types. The results, seen in Figure 1, demonstrate the specificity, durability, and uptake of MNPR-101-Zr in the metastatic tumors relative to normal tissue. The regions of higher uptake also align with the locations of the previously observed metastatic tumors on conventional FDG PET imaging.
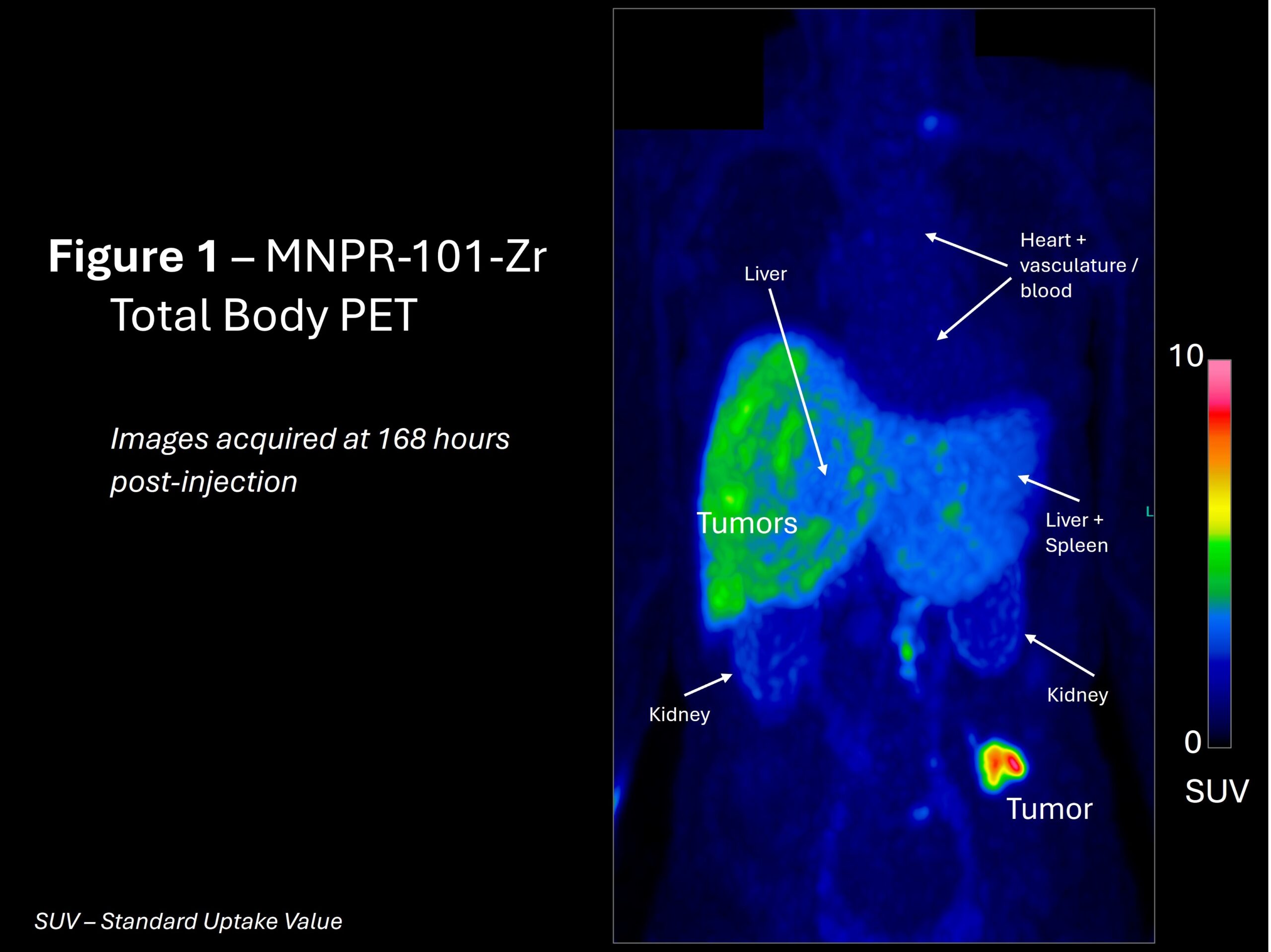
“This is exactly what we had hoped to see – highly preferential uptake in the tumor,” said Andrew Cittadine, Monopar’s Chief Operating Officer.
MNPR-101-Zr was evaluated against FDG, the gold standard for detecting metastatic tumors. Figure 2 shows FDG uptake in its highest-uptake tumor compared to MNPR-101-Zr uptake in the same tumor imaged on the same Siemens Biograph Vision Quadra PET/CT scanner.
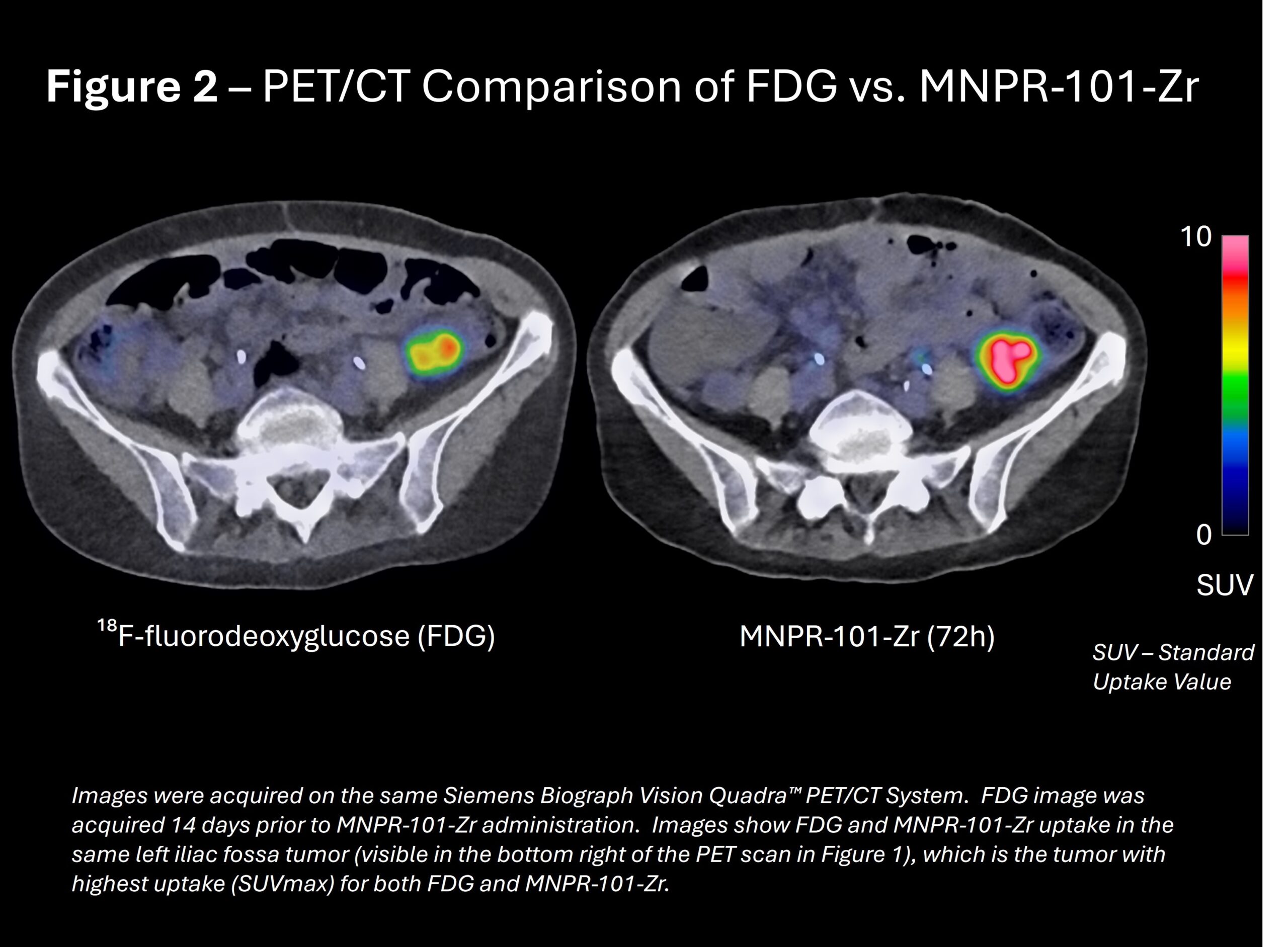
“At the Melbourne Theranostic Innovation Centre, we utilize one of the world’s most sensitive PET/CT scanners. Using the same scanner for FDG and MNPR-101-Zr, the results show MNPR-101-Zr achieved uptake at sites of known disease with retention out to late points, which is promising for future therapeutic translation,” said Professor Rodney Hicks, MBBS(Hons), MD, FRACP, FICIS, FAAHMS, lead investigator on the MNPR-101-Zr Phase 1 imaging and dosimetry clinical trial.
Monopar recently received clearance in Australia to initiate an MNPR-101-Lu Phase 1 therapeutic clinical trial [link] which is currently scheduled to launch in the fourth quarter of this calendar year.
“We are looking forward to sharing additional data at the upcoming European Association of Nuclear Medicine 2024 Annual Congress to be held in Hamburg, Germany on October 19-23, 2024, where our abstract has been accepted as a ‘Top-Rated Oral Presentation’ within the Scientific Program,” said Chandler Robinson, MD, Monopar’s Chief Executive Officer.
Further information about the ongoing MNPR-101-Zr Phase 1 imaging and dosimetry clinical trial is available at www.ClinicalTrials.gov under study identifier NCT06337084.
Monopar Therapeutics is a clinical-stage radiopharmaceutical company focused on developing innovative treatments for cancer patients, including Phase 1-stage MNPR-101-Zr for imaging advanced cancers, Phase 1-stage MNPR-101-Lu and late preclinical-stage MNPR-101-Ac225 for the treatment of advanced cancers, as well as early development stage programs against solid cancers. For more information, visit www.monopartx.com.
Total Page Views: 888









