BriaCell Reports 100% Resolution of Brain Metastasis in Breast Cancer Patient With “Eye-Bulging” Tumor
BriaCell Therapeutics Corp. reports significant anti-tumor response including complete resolution of temporal lobe breast cancer metastasis in a patient treated in the Phase 2 study of BriaCell’s Bria-IMT plus an immune checkpoint inhibitor regimen. The patient demonstrated an initial partial response at 2 months in the brain lesion with no detectable disease following 8 and 11 months of treatment.
The heavily pre-treated patient who had failed 8 prior regimens including ADC therapy, previously demonstrated significant reduction of her “Eye-Bulging” orbital tumor and continues treatment with the Bria-IMT regimen. She has completed 17 cycles of treatment and has been on BriaCell’s Phase 2 study for 12 months. Also noteworthy is a sustained drop in her tumor markers, confirming the imaging results of marked tumor reduction.
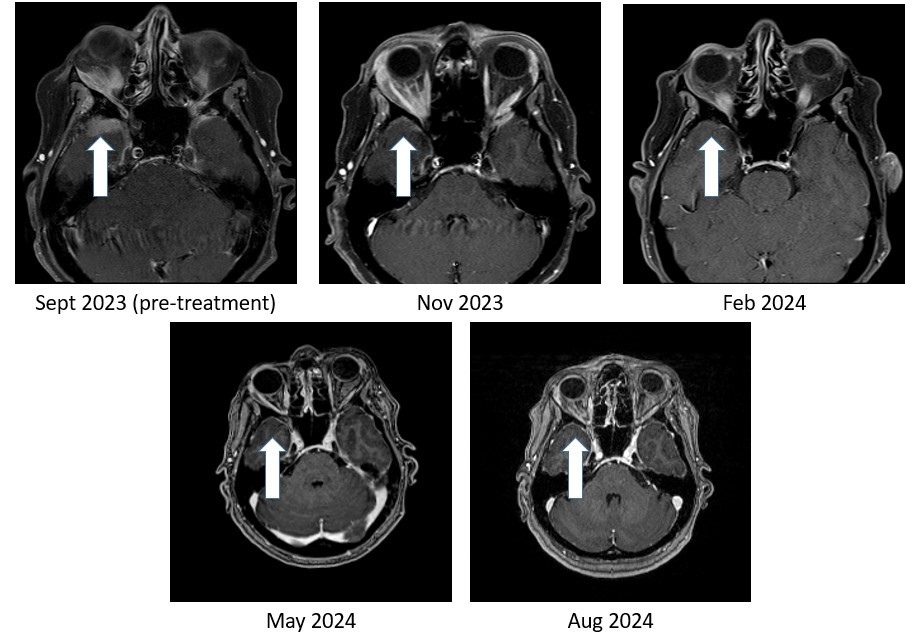
Figure 1. Bria-IMT regimen resulted in 100% resolution of tumor in the right temporal lobe region of the brain.
As shown in Figure 1, the right temporal lobe lesion is no longer detectable on the images taken at 8 months and 11 months on the Bria-IMT combination regimen. The orbital lesion has continued to shrink markedly (Figure 2). In addition, her tumor markers (blood tests that correlate with the amount of tumor in the body) remain markedly decreased from her pre-treatment levels.
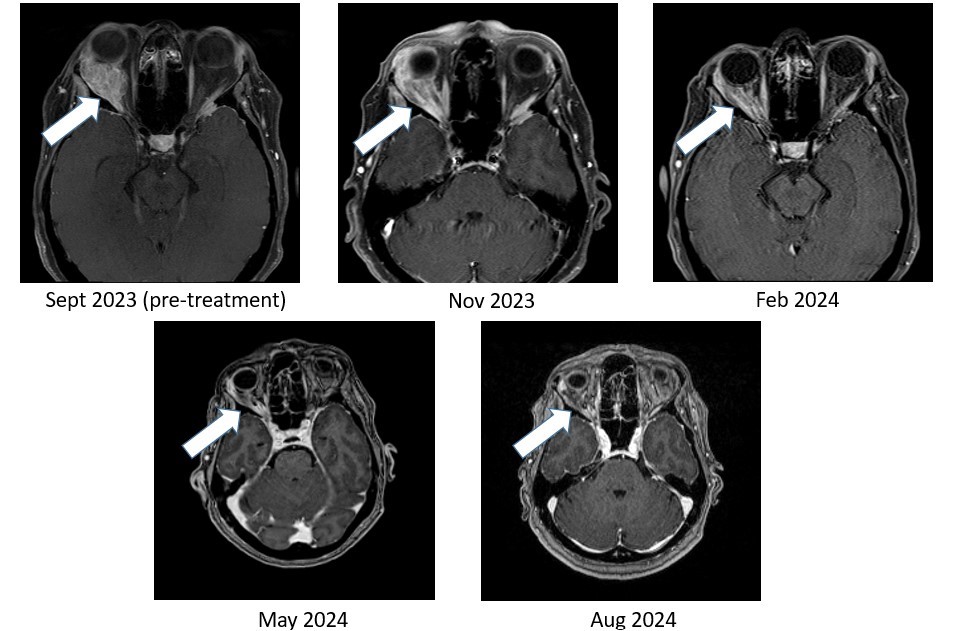
Figure 2. Bria-IMT regimen resulted in near complete resolution of breast cancer tumor in the right orbit (behind the eye).
“Bria-IMT’s potential therapeutic impact is unprecedented in metastatic breast cancer (MBC) in a brain metastasis setting. Our clinical findings, demonstrating significant tumor shrinkage in metastatic brain legions, may transform the way we treat MBC patients with brain metastasis, and offers hope to cancer patients and their families fighting this devastating disease,” stated Dr. William V. Williams, BriaCell’s President and CEO. “These results support Bria-IMT™ as a potential new therapeutic option for MBC patients with brain metastasis. We look forward to evaluating the brain metastasis patient subgroup in our ongoing pivotal Phase 3 study in metastatic breast cancer.”
“We believe that the complete tumor resolution in this patient with brain metastasis, plus other cases of significant anti-cancer clinical responses in our Phase 2 MBC patients with brain metastasis, highlight the potential application of Bria-IMT in treating similar MBC patients,” added Dr. Giuseppe Del Priore, BriaCell’s Chief Medical Officer. “The protracted time on therapy, now one year, attests to the excellent tolerability of the Bria-IMT regimen in combination with an immune checkpoint inhibitor which is being used in our pivotal Phase 3 study.”
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. For more information, visit https://briacell.com/.
Total Page Views: 805









