Issue:April 2014
BIOAVAILABILITY ENHANCEMENT - Diffusion of Innovation & the Adoption of Solubilization Technologies
Adoption of new technology, even when it has obvious advantages, is a difficult undertaking. Like everyone else writing in English, I am typing this column on a QWERTY keyboard. Have you ever wondered how we all ended up with this particular layout? As you might expect, there are other designs that are much easier to learn and allow much faster typing with lower error rates. In fact, the QWERTY keyboard was specifically designed by Christopher Sholes in the 1870s to slow down the typing rate so as to not jam the keys on a mechanical typewriter.1
With the early design of typewriters, this had the effect of actually speeding up the typing process because one didn’t have to deal with the jammed keys. However, as the mechanical design of typewriters improved and the typing style evolved to touch typing instead of hunting and pecking (for most of us), there was a significant driver to move away from the QWERTY design. In the 1930s August Dvorak, a professor at the University of Washington, introduced a keyboard design that took advantage of the strengths of the fingers and the statistical probabilities of which keys are most commonly typed. No matter the technological advantages of Dvorak’s keyboard, the design never gained popularity, and if you are in the market for a new keyboard, your local electronics store will be stocked only with the QWRETY version – a result that the famed economist W. Brian Arthur termed “lock-in.”2
Modern, complex technologies often experience rapid, exponential growth rates. Arthur’s hypothesis for this phenomenon was published in the 1980s with his pioneering theory of increasing returns. In short, the theory states that the adoption of a technology has an inherent positive feedback mechanism wherein the more a platform is adopted, the more users gain experience, and this adoption proliferation drives improvements in the technology.
According to Everett M. Rogers, the process of adoption of a new technological innovation follows a process in which the innovation is first made and then communicated via a particular medium to a network of potential adopters over a period of time.2
Even with rapid growth of a new technology, the users do not all adopt the innovation at the same time. At this point, I feel compelled to insert another thought critical to the topic at hand: “If you don’t change direction, you may end up where you’re heading.” – Lao Tzu (Zhou Dynasty)
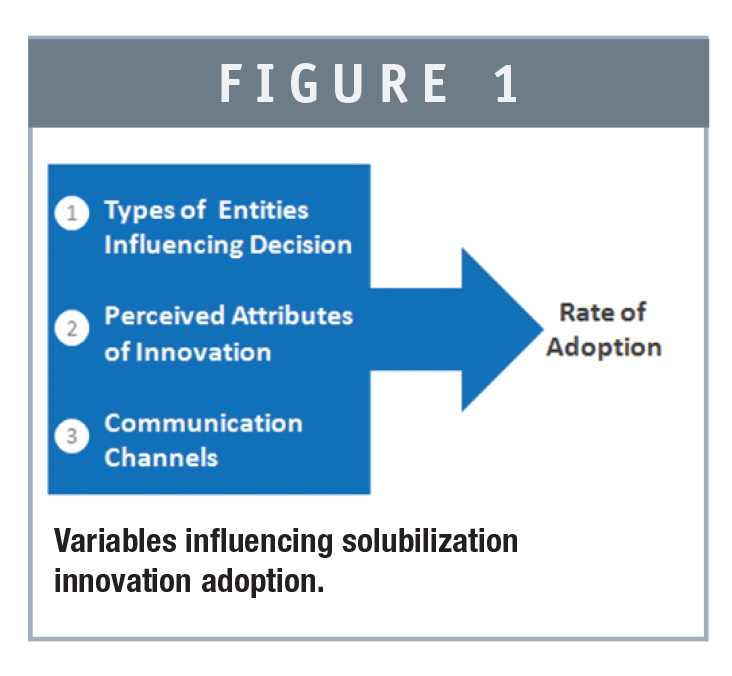 Whereas Pope’s admonition is to “play it safe,” 20 centuries earlier, Lao Tzu pointed out that change is essential if a different destination is desired (or required). Rogers acknowledges change agents in the first two of five groups of adopters, the Innovators and Early Adopters.1 The first group is obviously composed of the Innovators, individuals that recognize the need to solve a particular problem, who find the solution and then present it to those that have the need. At this point, a risk-taking group, referred to as the Early Adopters, latches on to the technology and applies it to a problem they have identified and that stands in their way toward progress. Given the success of this group, others take notice of the benefits of the innovation and recognize that much of the risk has now been removed due to the improvements made by the Early Adopters. Ultimately even the risk-averse become convinced the technology has merits and has been “de-risked” so they adopt the technology. The lifecycle of the technology continues toward maturation as the market becomes saturated. (Pope’s recommended stages would most likely be compatible with Early Majority and Late Majority users.)
Whereas Pope’s admonition is to “play it safe,” 20 centuries earlier, Lao Tzu pointed out that change is essential if a different destination is desired (or required). Rogers acknowledges change agents in the first two of five groups of adopters, the Innovators and Early Adopters.1 The first group is obviously composed of the Innovators, individuals that recognize the need to solve a particular problem, who find the solution and then present it to those that have the need. At this point, a risk-taking group, referred to as the Early Adopters, latches on to the technology and applies it to a problem they have identified and that stands in their way toward progress. Given the success of this group, others take notice of the benefits of the innovation and recognize that much of the risk has now been removed due to the improvements made by the Early Adopters. Ultimately even the risk-averse become convinced the technology has merits and has been “de-risked” so they adopt the technology. The lifecycle of the technology continues toward maturation as the market becomes saturated. (Pope’s recommended stages would most likely be compatible with Early Majority and Late Majority users.)
How can we apply this thinking to the growth of solubilization technology in pharmaceutical drug delivery? In this column, I’ll focus on one technology, spray-drying. As shown in the Second Quadrant column in the January/February 2014 issue of Drug Development & Delivery, solid dispersion technology was first applied in the 1990s. The cumulative number of approved drugs that use the technology is approximately 20. While it is clear the technology is being adopted at a more rapid rate in the past few years, it is interesting to consider the factors that are governing this growth. Table 1 lists a few of the drivers shaping the adoption rate of solid dispersion technology.
Starting with the obstacles to change, I believe it is fair to say that one of the major factors preventing widespread adoption of solid dispersion technology is the early on perception of inherent risk in the technology for pharmaceutical applications, and the pharmaceutical industry’s culture of caution. And no wonder there is trepidation when the path from discovery into the clinic can be perceived as already treacherous and somewhat unpredictable. Second to that, there are a number of key drivers that slow the adoption rate, including competition from alternative delivery platforms (namely lipid vehicles), the incremental added cost of development, and the significant capital investment required to deploy the requisite spray-dry equipment. In addition, the relative lack of experience and formulation expertise with solid dispersions influences the selection of the technology at early stages due to the perceived complexity of the technology.

As for factors driving the adoption of solid dispersion technology, it goes without saying that the number one reason is the increasing numbers of poorly soluble molecules coming from discovery. But many other factors exist. For example, the commercial success of companies such as Vertex stands out as a leading example of what can be accomplished with the technology. However, equally important are the continued innovation in the field and the rapidly growing, collective knowledge base in the industry.
As a measure of the industry knowledge in the solid dispersion space, we at Agere analyzed the number of literature citations that include “solid dispersion” and “pharmaceutical.” Figure 2 shows the results of this analysis, and that the literature in the field is growing at an exponential rate. Further analysis of this curve leads one to conclude that we as an industry are somewhere in the Early Majority category of adopters. This implies that the utilization of solid dispersions has many years left before the technology becomes mature.
Assuming that poorly soluble molecules are here to stay, and that history repeats itself, I have no doubt that eventually new innovations or completely new technologies will emerge that will eclipse current solutions. I’m curious about when and what these might be.
To view this issue and all back issues online, please visit www.drug-dev.com.
REFERENCES
1. For a more complete description, see Diffusion of Innovations, 5th Ed., Everett M. Rogers, 2003 ISBN 978-0-7432-2209-9.
2. Arthur BW. Competing technologies, increasing returns, and lock-in by historical events. The Economic Journal. 1989;99(394):116-131.
3. Agere analysis.

Marshall Crew
President & CEO
Agere Pharmaceuticals, Inc.
crew@agerepharma.com
LinkedIn: https://www.linkedin.com/ profile/view?id=17815140
Total Page Views: 1788


















