Aptar CSP Technologies’ N-Sorb Nitrosamine Mitigation Solution Accepted to US FDA’s Emerging Technology Program
Aptar CSP Technologies recently announced its N-Sorb nitrosamine mitigation solution has been accepted into the US FDA’s Emerging Technology Program (ETP), which helps promote the adoption of innovative approaches to pharmaceutical product design and manufacturing.
N-Sorb leverages Aptar CSP Technologies’ proven 3-Phase Activ-Polymer platform technology to address the pressing issue of N-nitrosamine impurities in pharmaceuticals. These impurities, classified as probable human carcinogens, have raised significant regulatory concerns and prompted numerous drug product recalls. Nitrosamines can form during drug product storage or transport, posing risks to patient health.
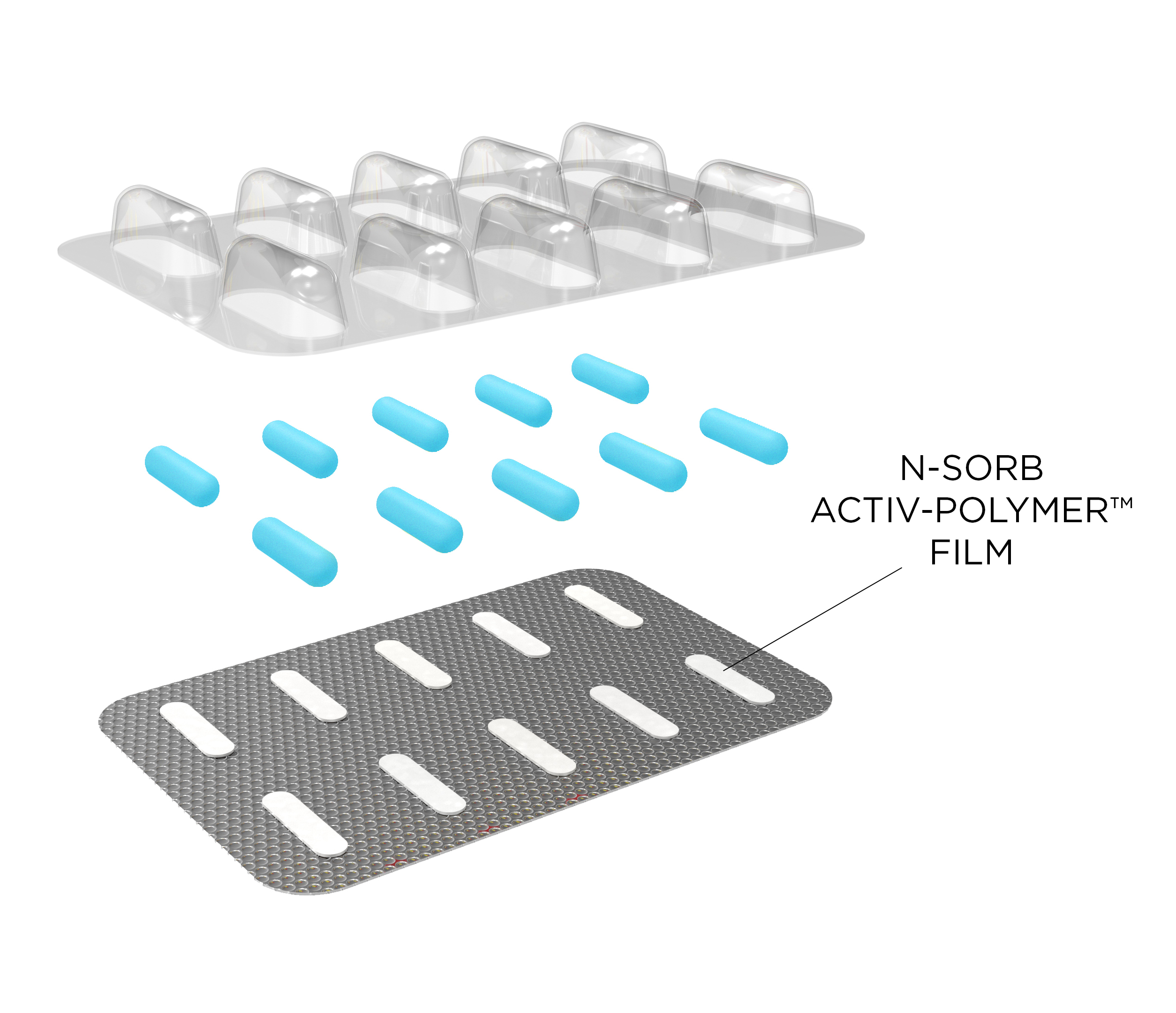
Aptar CSP Technologies’ N-Sorb Nitrosamine Mitigation Solution Deployed as Activ-Blister™
N-Sorb technology can be deployed in multiple formats by integrating active material science into polymers. For example, the technology can be incorporated into a blister format that integrates a piece of N-Sorb Activ-Film material into each individual Activ-Blister cavity. This same platform is currently trusted by global brands to protect sensitive APIs from degradation due to moisture and oxygen exposure. Alternatively, the technology can be seamlessly integrated into a container closure system. N-Sorb’s intelligent design allows it to react with nitrosamine precursors in the packaging headspace to inhibit nitrosamine formation and scavenge nitrosamine impurities post-formation.
By delivering this Generally Recognized as Safe (GRAS) material directly within the packaging, N-Sorb can eliminate the need for pharmaceutical developers to reformulate their drug products, which could support compliance with US FDA and EU EMA regulations regarding safe nitrosamine levels. The active packaging intervention represents a paradigm shift in managing impurities and degradation, which could significantly enhance overall mitigation strategies and aligns with the latest FDA guidance (updated Sept. 4, 2024) that recognizes packaging changes as a potential mitigation strategy. By addressing nitrosamine concerns with active packaging, N-Sorb technology can help accelerate drug product development and help alleviate the burden of drug shortages due to recalls.
“The FDA’s Emerging Technology Program is highly selective, reserved for the most promising pharma and healthcare sector solutions,” said Badre Hammond, VP Global Commercial Operations and GM for Aptar CSP Technologies. “Our ability to mitigate nitrosamine formation with active material science introduces a critical quality control element designed to ensure patient safety. We are eager to collaborate with the FDA’s Emerging Technology team to empower pharma brands with this innovative offering.”
As part of the program, industry representatives meet with the FDA’s Emerging Technology Team members to discuss, identify and resolve potential technical and regulatory issues related to the development and implementation of novel technologies prior to regulatory submission. This multi-stakeholder effort aligns with the FDA’s mission to facilitate modernization in the pharmaceutical industry, reducing time and cost requirements for introducing novel solutions.
Aptar CSP Technologies is part of AptarGroup, Inc., a global leader in drug and consumer product dosing, dispensing, and protection technologies. Aptar CSP Technologies leverages its active material science expertise to transform ideas into market opportunities, accelerate and de-risk the product development process, and provide complete solutions that improve consumers’ and patients’ lives. The company offers a complete set of services from concept ideation, to design and engineering, to product development, global production, quality control, and regulatory support that results in expedited speed-to-market. For more information, visit www.csptechnologies.com and www.aptar.com.
Total Page Views: 726









