ACELYRIN Announces Positive Phase 1/2 PoC Data for First Subcutaneous Anti-IGF-1R to Demonstrate Clinical Responses in Thyroid Eye Disease
ACELYRIN, INC. recently announced positive proof-of-concept data from an ongoing Phase 1/2 trial of lonigutamab in thyroid eye disease (TED). Lonigutamab is a subcutaneously (SC) delivered humanized IgG1 monoclonal antibody targeting the insulin-like growth factor-1 receptor (IGF-1R), a validated mechanism of action for the treatment for TED.
In the Phase 1/2 trial, lonigutamab demonstrated rapid improvements in proptosis and clinical activity score (CAS) at the first measurement – within 3 weeks after the first subcutaneous dose.
“These results are the first reported clinical data for the anti-IGF-1R mechanism delivered subcutaneously and demonstrating clinical benefit in thyroid eye disease patients. The data support our hypothesis that lonigutamab has the potential to optimize benefit-risk by enabling longer-term subcutaneous dosing to increase depth and durability of clinical response while attempting to limit safety liabilities by avoiding the high maximal concentrations resulting from IV administration, while maintaining optimal therapeutic levels,” said Shao-Lee Lin, MD, PhD, Founder and CEO of ACELYRIN. “Tackling thyroid eye disease has special meaning for our team, and we are thankful to the patients and investigators who have partnered with us. We are delighted to have achieved proof of concept for lonigutamab in TED and intend to advance clinical development with the potential to move patients toward resolution of their disease.”
This multi-center, dose-ranging Phase 1/2 clinical trial is evaluating the safety and efficacy of lonigutamab dosed in TED patients. Cohort 1 was placebo-controlled with six patients receiving lonigutamab and two receiving placebo. Cohort 2 is open label with data available from six patients at 6 weeks.
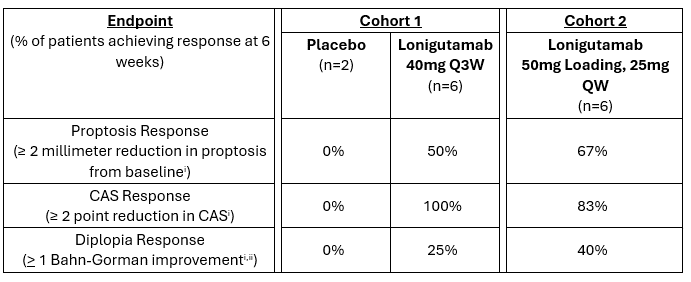
i Clinically meaningful results across each measurement
ii Teprotumumab Smith, et al NEJM 2017 21% placebo-adjusted rate at 6 weeks; Douglas, et al NEJM 2020 39% placebo-adjusted rate at 24 weeks
Overall, lonigutamab has been well-tolerated across our clinical experience to date. There have been no reports of hyperglycemia or hearing impairment and no serious adverse events.
“It is very encouraging to see the results of subcutaneous administration of an anti-IGF-1R therapy. The data shown suggest that there is a clinically meaningful response in patients as early as 3 weeks after a single subcutaneous dose of lonigutamab. In addition, it appears that the safety profile of medication through the subcutaneous route may be favorable when compared to standard of care,” said Shoaib Ugradar, MD, Department of Orbital and Oculoplastic Surgery, private practice, Beverly Hills, California. “It is important to note that this is preliminary data in a small group, however the positive results are highly promising. Given the growing body of evidence that suggests thyroid eye disease may have long-term sequelae, the convenience of a subcutaneous administered medication with a potentially favorable side effect profile becomes critical.”
With proof of concept achieved in Cohort 1, and Cohort 2 further validating these results, a Phase 2b/3 trial is planned to be initiated in the second half of 2024, designed to be the first of two registrational trials in TED.
Given the close proximity of the recent data announcements for izokibep and lonigutamab and today’s conference call, ACELYRIN will forego hosting a fiscal year 2023 earnings call. The company will instead announce its financial results in a press release and file the related 10-K report no later than April 1, 2024.
The Phase 1/2 clinical trial (NCT05683496) is a multi-center trial evaluating the safety and efficacy of lonigutamab dosed subcutaneously in three cohorts of patients with active thyroid eye disease (TED). Cohort 1 is placebo-controlled testing lonigutamab 40mg every 3 weeks (Q3W) through 6 weeks, cohort 2 is open label testing a 50mg loading dose followed by 25mg every week (QW), and cohort 3 is testing every four weeks (Q4W) dosing.
For more information about the Phase 1/2 trial, please visit www.clinicaltrials.gov.
Thyroid Eye Disease (TED) is a vision-threatening autoimmune disease in which there is both inflammation and expansion of the tissues behind the eye, resulting in eye bulging, known as proptosis, and the subsequent inability to close the eyelids. Double vision, or diplopia, can occur, as well as the potential for compression of the retinal nerve, which can lead to blindness. Thus, TED is a progressive, chronic inflammatory disease where longer-term treatment has the potential to improve depth and durability of response. More than 100,000 people in the United States are estimated to suffer from TED.
Lonigutamab is a humanized IgG1 monoclonal antibody targeting the IGF-1 receptor and is delivered subcutaneously. Relative to standard of care, lonigutamab binds to a distinct epitope, which results in internalization of the receptor within minutes, and in preclinical binding and functional laboratory assays, it has been shown to be 75-fold more potent. The characteristics of lonigutamab that enable subcutaneous delivery also enable the potential for longer-term dosing, which we believe can improve depth and durability of clinical response. Based on our preclinical and pharmacodynamic data from our completed single ascending dose study with lonigutamab, we can optimize the therapeutic window utilizing the SC route of administration. The characteristics of lonigutamab also allow the potential to minimize exposures relative to IV therapy. IGF-1 is neuroprotective to cochlear cells of the inner ear and serves to repair damage that can occur over time. We hypothesize that high concentrations of anti-IGF-1R due to Cmax from IV administration can penetrate the blood-labyrinth barrier and interfere with this normal function. Lonigutamab originated from Pierre Fabre Laboratories, a French pharmaceutical group.
ACELYRIN, INC. (Nasdaq: SLRN) is a Los Angeles area-based late-stage clinical biopharma company – with additional operations in the San Francisco Bay area – focused on providing patients life-changing new treatment options by identifying, acquiring, and accelerating the development and commercialization of transformative medicines. ACELYRIN has two programs in late-stage clinical development. Izokibep is a next generation inhibitor of IL-17A in Phase 3 development for the treatment of psoriatic arthritis, hidradenitis suppurativa and uveitis. Lonigutamab is a subcutaneously delivered monoclonal antibody targeting IGF-1R being investigated for the treatment of TED. For more information, visit www.acelyrin.com.
Total Page Views: 1613









