Issue:November/December 2025
3D PRINTING - The Future of 3D Printing in Pharma: Aprecia’s Vision for Transformative Medicine
INTRODUCTION
As the pharmaceutical industry stands at the intersection of innovation and urgency, the need for transformative solutions has never been more critical. Global supply chain disruptions, rising demand for personalized therapies and the limitations of traditional manufacturing methods have exposed systemic vulnerabilities. Patients are waiting longer for essential medications and manufacturers are under pressure to deliver safer, more effective treatments with speed and precision.
Aprecia, a pioneer in pharmaceutical 3D printing, has a vision for distributed, patient-centric manufacturing that could reshape the future of medicine. With proprietary platforms that offer flexible oral dosage forms with tailored functionalities and a range of API compatibility, Aprecia is not just responding to industry challenges; it’s redefining how drugs are formulated, produced, and delivered.
The COVID-19 pandemic brought to light the limitations and challenges of global pharmaceutical supply chains. From active pharmaceutical ingredient (API) shortages to delays in packaging and distribution, the ripple effects were felt across continents. Even beyond the pandemic, climate-related disruptions and increasing regulatory complexity continue to strain traditional manufacturing models.
At the same time, the rise of personalized medicine driven by advances in diagnostics and patient data is demanding a shift from mass production to tailored therapies. Patients with rare diseases, pediatric populations, and those with complex dosing needs require medications that are effective and can be customized to their physiology and lifestyle.
Traditional manufacturing, with centralized facilities and rigid batch processes, can struggle to meet these evolving demands. The industry is waiting for a shift that embraces flexibility, precision and resilience.
3D PRINTING IS REVOLUTIONIZING MEDICINE
Aprecia’s response to these challenges is rooted in its mastery of binder jet 3D printing, a technology that enables the creation of oral solid dose medications with unprecedented control and customization.
- 3DP binder jetting technology allows for high-dose, fast-dissolving tablets that are beneficial for patients with swallowing difficulties, including children, the elderly, and those with neurological conditions.
- Precision formulation with 3DP development expertise builds on this by enabling tailored mechanical properties, taste masking, and enhanced bioavailability, all within a single dosage form.
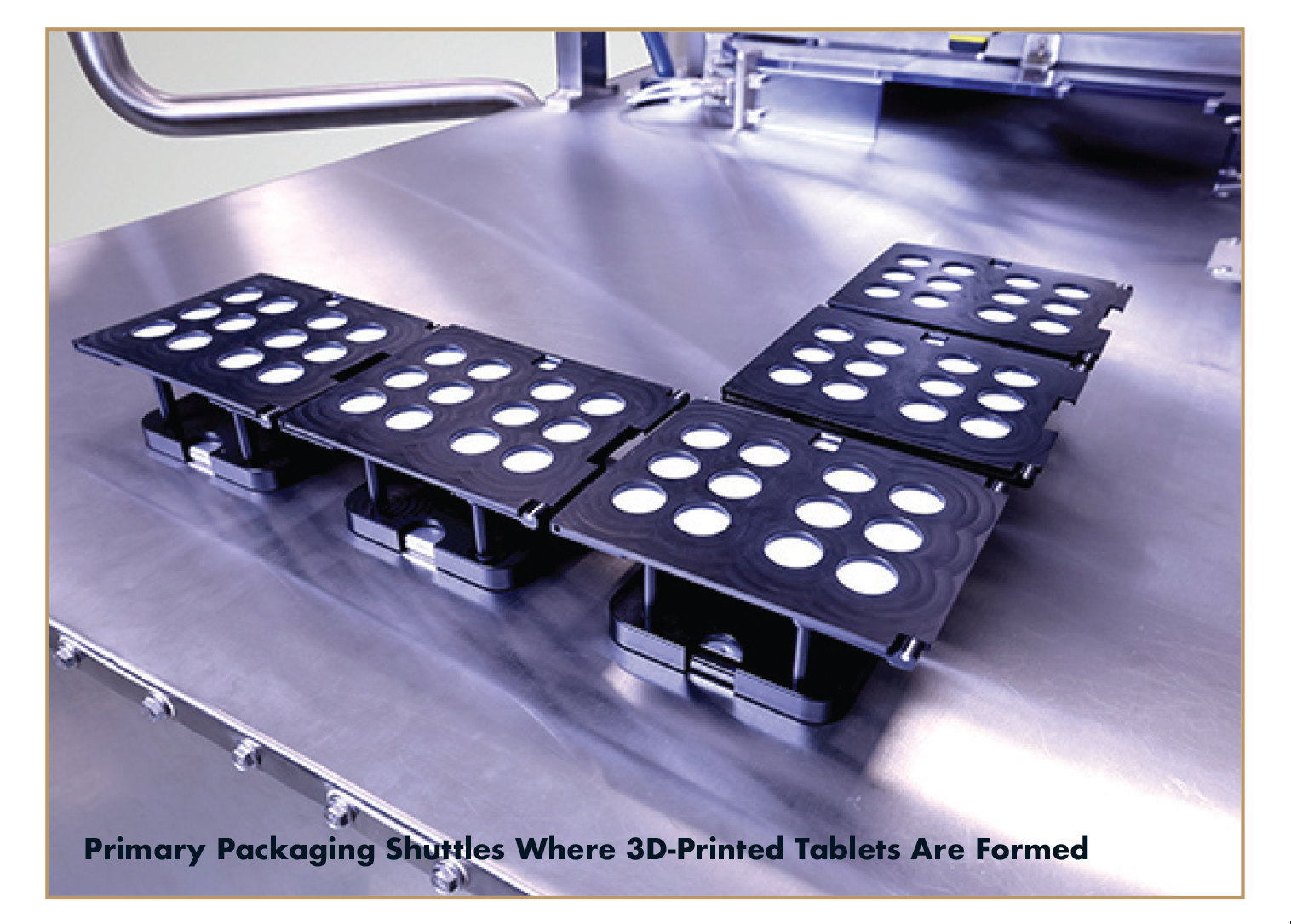
- At the heart of Aprecia’s innovation is its in-blister binder jetting platform that prints dosages layer-by-layer directly into blister packaging. This continuous manufacturing process supports real-time quality monitoring and batch release, eliminating the need for separate packaging steps and reducing production timelines.
The in-blister 3DP-Tablet Production Platform is designed for scalability without altering the manufacturing process or sacrificing rigor and precision. This flexibility supports distributed manufacturing, allowing production to occur closer to the point of care, reducing transportation costs and mitigating supply chain risks.
Aprecia’s distinct, patent-protected 3DP production platforms extend well beyond prototyping and are built for commercial-scale production. The company’s Open Bed racetrack-style 3D printing configuration delivers breakthrough binder jetting innovations that can deliver capacity at scale. Each platform can produce clinical supplies suitable for Phase I-III trials, accelerating development timelines and market entry.

APRECIA’S STATE-OF-THE-ART TECHNOLOGY
Aprecia’s state-of-the-art technologies prioritize speed, flexibility, and precision, setting new standards for reliable medication production and distribution. Aprecia’s technology takes a comprehensive approach to prioritize and deliver:
- Efficiency and Risk Reduction: Aprecia’s continuous ‘racetrack’ 3D printing configuration eliminates wait times, boosts efficiency and ensures precise medication dispensing while minimizing risk.
- Flexibility and Market Entry: Our pioneering 3DP technology platform optimizes development processes and expedites market entry.
- Prototyping and Clinical Supplies: In-Blister 3DP-Tablet production platform enables quick development of prototypes and early clinical supplies, reducing investment risk and allowing faster go/no-go decisions in clinical trials.
- Enhanced Clinical Trial Efficiency: Aprecia’s technology enhances clinical trial efficiency and cost-effectiveness, reducing delays and improving responsiveness to protocol changes.
- Proprietary Powder Handling Technologies: Precise powder and liquid deposition at clinical and commercial scales enable rapid design, prototyping and manufacturing of novel dosage forms.
- Accelerated Product Development: The technology accelerates product development through Phase 1 and 2 programs, streamlining product development and enabling rapid screening and elimination of non-viable prototypes.
For pharmaceutical partners, this means faster go/no-go decisions in clinical trials, reduced investment risk, and the ability to respond quickly to protocol changes.
THE ADVANTAGES OF ADVANCED DISTRIBUTED MANUFACTURING
Aprecia’s advanced platforms offer significant advantages in the realm of distributed manufacturing. These technologies enable pharmaceutical companies to manufacture complex tablet structures, providing the freedom and flexibility to design novel dosage formulations that address unmet patient needs.
- Complex Tablet Structures: Aprecia’s technology enables the manufacturing of tablets with varying degrees of hardness and mechanical strength, allowing for the creation of novel dosage formulations that incorporate embedded functionalities.
- Dosage Solutions: The platform solves common dosage challenges by producing pill structures that dissolve effortlessly in a patient’s mouth with just a sip of water, improving ease of administration and patient compliance.
- Customizable Medications: Aprecia supports the production of highly customizable, patient-specific medications with precise dosing, offering both cost and logistical advantages for pharmaceutical partners.
Flexible Administration: The system provides flexible oral administration options and dosage forms, including embedded features for taste masking and enhanced bioavailability, while maintaining broad API compatibility to meet diverse dosing needs.
Aprecia is setting a new benchmark for pharmaceutical innovation by combining speed, flexibility, and precision in commercial-scale 3D printing. Our platforms streamline formulation timelines, support real-time batch release and enable the rapid development of complex, patient-friendly dosage forms. By producing entirely in the US, we’re not only addressing supply chain vulnerabilities but also empowering pharmaceutical partners with on-demand, distributed manufacturing capabilities that unlock personalized medicine and accelerate clinical success.
REAL LIFE SUCCESSES
In 2015, Aprecia made history with Spritam®, the first FDA-approved 3D-printed medication used to treat partial-onset seizures in patients aged four and older. This milestone validated 3D printing as a viable pharmaceutical manufacturing method and opened the door for broader regulatory acceptance.
Since then, Aprecia has secured over 126 patents and continues to collaborate with regulatory bodies to advance agile manufacturing standards. Aprecia’s work with ZipDose® and other platforms demonstrates a commitment to meeting regulatory requirements but helping to shape and redefine them as well.
Over the past year, Aprecia has been collaborating with one of our strategic partners in a government funded research program to investigate how a drug substance synthesis platform, combined with Aprecia’s revolutionary solid oral dose 3D printing technology can accelerate US drug production to deliver high quality drug products. Under this program:
- Aprecia is developing two drug products for registration with the FDA which are categorized by the US government as essential medicines for the US population.
- Aprecia will produce both the active pharmaceutical ingredients and the finished pharmaceutical dosage forms at the same location, significantly shortening the pharmaceutical raw material supply chain while also lessening supply chain risk for commercial product distribution.
- Aprecia will generate key scientific data needed to demonstrate that agile manufacturing can meet FDA drug product registration requirements and be utilized to produce safe and effective pharmaceutical products.
These types of government research programs enable us to contribute toward important public health initiatives while facilitating broader adoption of advanced manufacturing technology, such as Aprecia’s 3D printing technology, unlocking private and public capital for commercialization of innovative pharmaceutical technology and propel the agile pharmaceutical manufacturing technology into the marketplace.
The potential impact of this work is far reaching. Aprecia will use agile pharmaceutical manufacturing technology to address critical drug shortages more rapidly, enable point-of-need manufacturing to support military operations and medical countermeasure applications, enhance the overall resilience of the pharmaceutical supply chain and develop the foundation for more personalized medicine success.
Aprecia’s technology also addresses common barriers to medication adherence. Tablets that dissolve effortlessly improve ease of administration, especially for patients with dysphagia or those requiring high-dose therapies. Customizable dosing ensures that each patient receives the exact amount needed, reducing side effects and improving outcomes.
The ability to embed functionalities, such as taste masking, controlled release, and multi-API combinations, within a single tablet offers pharmaceutical companies new avenues for innovation. These features are particularly valuable in pediatric and geriatric care, where traditional dosage forms often fall short.
BENEFITS BEYOND RELIANT & FAST MANUFACTURING & PRECISION DOSING
Distributed manufacturing not only enhances resilience but also reduces environmental impact. By decentralizing production, Aprecia minimizes transportation emissions and packaging waste. On-demand printing reduces overproduction and inventory costs, aligning with sustainability goals and lean manufacturing principles.
For pharmaceutical companies, this translates into lower operational costs, faster time to market and improved responsiveness to demand fluctuations.
Our innovative approach to medicine production not only addresses current supply chain vulnerabilities but also supports efforts to widen manufacturing capabilities in the US. Aprecia manufactures pharmaceuticals solely in the US, and our platform streamlines manufacturing in two keyways. First, it enables us to create unique products that conventional technologies can’t — like SPRITAM and a range of other dosage forms from orally disintegrating tablets to extended-release and suspension formats. Second, our process supports distributed and flexible manufacturing, which opens the door to onshoring, personalized medicine and on-demand production. We’ve shown that 3D printing isn’t just for prototyping — it’s a viable commercial manufacturing solution.
The global market for pharmaceutical 3D printing is projected to grow significantly over the next decade, driven by demand for personalized medicine and advanced manufacturing technologies.
LEADING THE FUTURE OF PHARMACEUTICAL PRODUCTION
Aprecia is the only advanced additive manufacturer with an FDA-approved product and commercial-scale capabilities.
With a focus on oral solid dose medications, combined with deep expertise in formulation science and regulatory strategy, Aprecia is a category leader. Competitors may offer prototyping tools or niche applications, but Aprecia’s end-to-end platform — from development to distribution — sets it apart.
As we look ahead, Aprecia’s commitment to innovation, speed, and precision positions it at the forefront of pharmaceutical transformation. Its platforms enable flexible, localized production models that support onshoring, personalized therapies, and rapid response to emerging health needs.
3D printing is no longer a future promise — it’s a present-day solution. At Aprecia, we’ve proven that additive manufacturing can deliver scalable, patient-centric therapies with speed, precision and resilience. Our platforms are built not just to meet today’s challenges, but to redefine what’s possible in medicine tomorrow.
By combining advanced 3D printing with strategic partnerships and regulatory leadership, Aprecia is not just changing how medicine is made — it’s changing what is possible.
TRANSFORMING PHARMA WITH SCALABLE 3D PRINTING
The pharmaceutical industry must evolve to meet the demands of a changing world. Aprecia’s vision for distributed, patient-centric manufacturing offers a blueprint for that evolution. With proven technology, regulatory milestones and a track record of innovation, Aprecia invites pharmaceutical partners, policymakers, and healthcare providers to join in shaping the future of medicine.

Kyle Smith is President & COO of Aprecia Pharmaceuticals, where he leads strategic operations and innovation in 3D-printed drug manufacturing. With over a decade at Aprecia, he’s held roles from Operations Engineer to VP of Operations, driving advancements in pharmaceutical technology and GMP compliance. He also serves on the Board of Directors at BAMF Health, a leader in cancer imaging and theranostics. He earned a Chemical Engineering degree from Georgia Tech and an MBA from Miami University.
Total Page Views: 3934













