Issue:November/December 2025
ORAL SUSPENSIONS - A New Standard in Oral Suspensions: Leveraging Novel Excipients for FDA Approval
WHEN ORAL SUSPENSIONS ARE THE ONLY CHOICE
Oral administration is the most common route of delivery for many therapeutic agents due to its convenience, ease of use, and non-invasiveness.1 Liquid dosage forms are often the ideal solution to accommodate diverse patient groups such as pediatrics, geriatrics, and up to 16% of the general population with dysphagia.2 Swallowing difficulties are also prevalent in patients with disorders resulting from various conditions like stroke or neurological diseases. In severe cases, these conditions may require enteral feeding via nasogastric (NG) or percutaneous endoscopic gastrostomy (PEG) tubes. In these settings, an oral suspension is not just a matter of preference but often the only viable means of ensuring that patients continue to receive their medications safely and consistently.
With nearly 90% of drug entities being poorly water-soluble, addressing solubility and bioavailability is a critical priority in drug development.3 Dispersing the drug is one of the most direct approaches to dealing with a poorly soluble active pharmaceutical ingredient (API). However, this creates a new set of complex challenges. A successful suspension demands careful consideration of chemical and physical stability, palatability for oral administration, accurate and flexible dosing, and precise in-vitro dissolution. Viscosity especially plays a vital role for patients requiring enteral feeding, as it directly impacts both administration and drug delivery.
This article introduces a new approach to perfecting oral suspension formulation. It is centered on the strategic use of a “novel” synthetic functional excipient, magnesium aluminometasilicate (MAS). We explore its unique properties, its successful journey through the FDA approval process, and its application in a real-world case study on NSAIDs. By showcasing how we successfully navigated the challenges of working with a novel excipient and a complex suspension system, we aim to provide formulators with a clear pathway to overcome their most difficult challenges and drive innovation in drug development. Ultimately, our work illustrates how the deliberate choice of excipients can transform an otherwise limited formulation into a patient-friendly product with commercial longevity.
WHY MAGNESIUM ALUMINOMETASILICATE?
MAS possesses unique functional properties that were essential for the success of our oral suspension development. Its high surface area and porous structure enable controlled dissolution rate, API dispersion, and amorphous stabilization, while its chemical composition provides additional benefits for stability and its potential to indirectly contribute to taste masking. These properties directly address the most common challenges encountered when moving from early formulation screening to late-stage development.
Their impact will be further detailed in our case study, which highlights how MAS was successfully incorporated into our FDA approved oral suspension product. The decision to include this novel material in our formulation was driven by its functional advantages, despite the complexity of the regulatory pathway. This approved product supports our formulation science and regulatory strategy to achieve a new standard in oral suspension delivery.
THE CHALLENGE & OPPORTUNITY OF A NOVEL EXCIPIENT
In the eyes of the FDA, an excipient is considered “novel” when it has not been previously used in an approved drug product in the U.S. for the intended route and dosage level.4 A novel excipient often differs significantly from established excipients, which are typically natural or semi-synthetic. MAS fits this definition because it is a synthetic compound, unlike its counterpart, Magnesium Aluminum Silicate, which is naturally derived from purified smectite clays.5
The novelty of MAS and other synthetic materials can create confusion in the public domain. For instance, there are often confounding CAS numbers and misleading descriptions of natural and synthetic materials as equivalent. This lack of clarity and the absence of a defined regulatory pathway for such materials highlights the need for a rigorous strategy. While synthetic materials like MAS are often valued for their high purity and lot-to-lot consistency, they come with high regulatory barriers, which often discourage pharmaceutical companies from using excipients beyond those already approved. As a result, the adoption of novel excipients lags significantly behind scientific discovery, creating a gap between what is technically possible and what is practically implemented in commercial products.
Securing approval for a novel excipient is a rigorous and data-intensive process. Novel excipients are not listed on the FDA’s Inactive Ingredient Database (IID), so their safety and performance must be thoroughly demonstrated through formal regulatory submissions. Our team successfully navigated this pathway by collaborating closely with the manufacturer/supplier of MAS, who submitted a detailed Drug Master File (DMF) to the FDA. We provided expert input and conducted a thorough review of the open portion of the DMF, which contained comprehensive data on chemistry, manufacturing, and controls. An extensive toxicological evaluation and the full toxicokinetic behavior of other MAS grades and related compounds were also presented to justify the safety and effectiveness of this excipient, leading to a positive outcome and regulatory acceptance of the material. The FDA’s formal acknowledgment of MAS’s approvability was a key milestone, demonstrating our expertise in managing complex regulatory processes and our ability to incorporate novel materials into new drug formulations.
A CASE STUDY: FORMULATING AN NSAID SUSPENSION
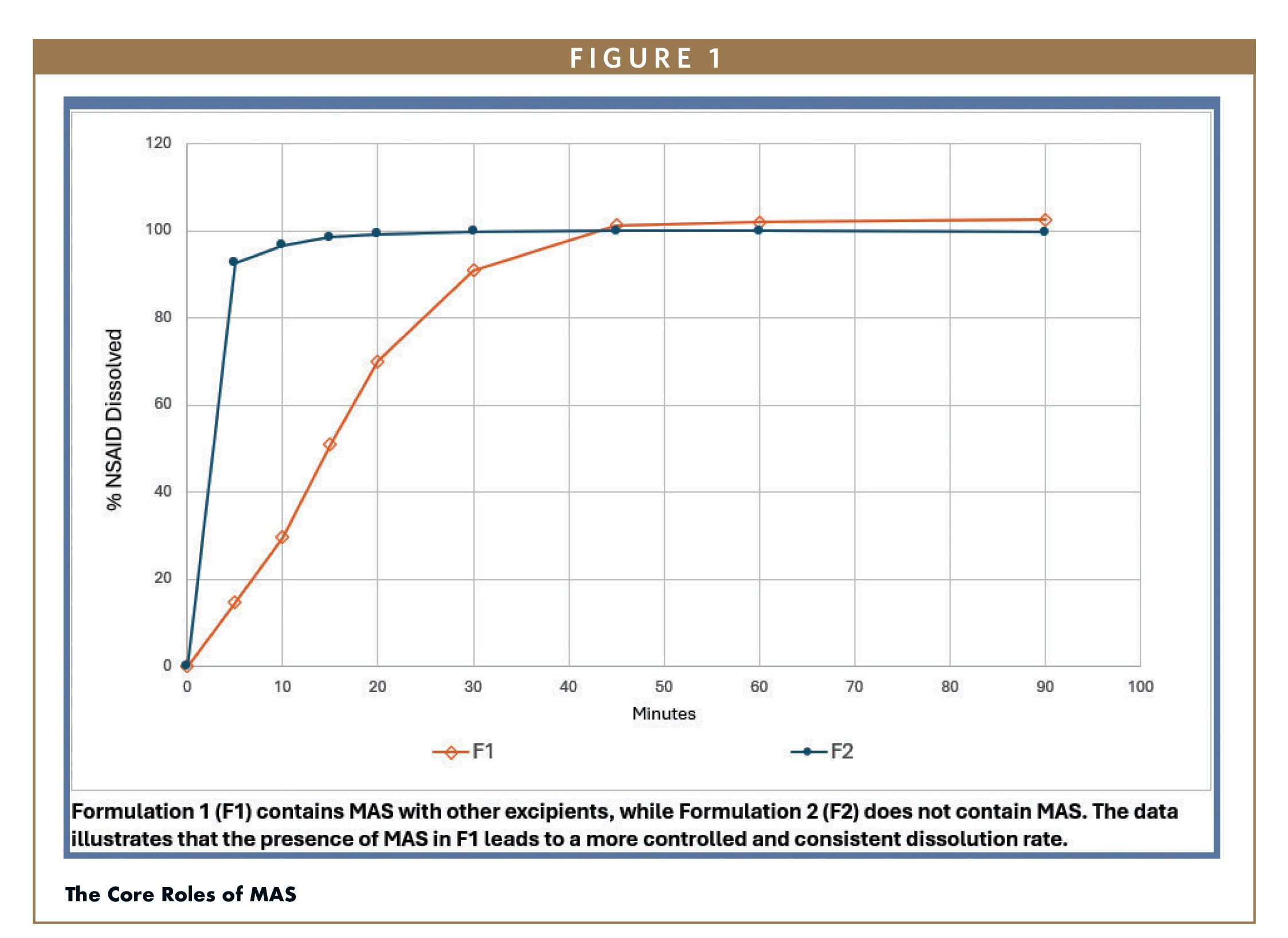
The Core Roles of MAS
The unique challenges of formulating select NSAIDs, which are known for their poor solubility and bitter taste, provide a compelling use case for a novel excipient like MAS. Its high surface area (300 m3/gm) and porous structure facilitate the adsorption of the API at a near-molecular level, which enhances dispersion and prevents particle aggregation, thereby maximizing the drug’s exposure to gastrointestinal fluids and leading to a more consistent dissolution profile. Furthermore, MAS helps stabilize the amorphous form of the drug, preventing recrystallization and maintaining the controlled dissolution properties. For these NSAIDs, this stabilization can be the difference between a formulation that delivers a consistent and reproducible release profile and one that fails to achieve reliable results.
Beyond its impact on drug performance, MAS contributes significantly to product stability and patient acceptability. A key quality attribute of elegant suspensions is redispersibility: the ability to be easily and homogeneously re-dispersed with gentle shaking after sedimentation. The highly porous nature of MAS slows settling and caking of suspended drug particles, ensuring dose uniformity throughout the product’s shelf-life. Its ability to effectively encapsulate drug particles and promoting fine, uniform dispersion, also indirectly contributes to taste masking. This barrier formation can shield direct contact with taste receptors in the mouth, and the improved drug dispersion can prevent localized high concentrations of bitter drug in the suspension and potentially help reduce the need for extensive flavor work. In pediatric populations especially, palatability is often the single largest driver of adherence, and the potential for MAS to help minimize bitterness offers a practical route to better compliance without relying solely on flavoring agents or sweeteners.
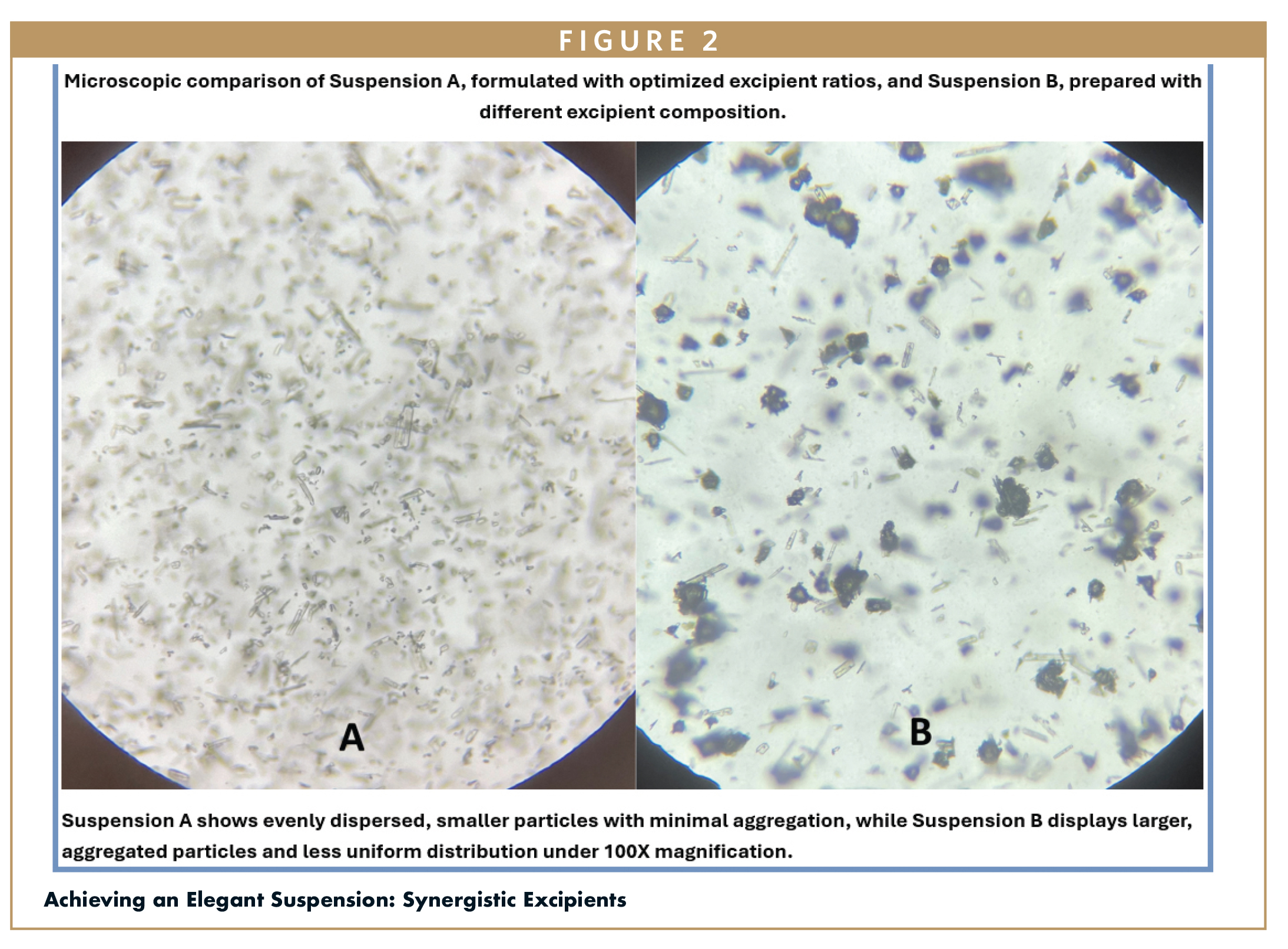
Achieving an Elegant Suspension: Synergistic Excipients
By combining MAS with other carefully selected functional excipients, we optimized the in-vitro dissolution profile while improving overall patient experience. For example, xanthan gum (XG), a key rheology modifier, was crucial for providing long-term physical stability. When dispersed in an aqueous solution, XG forms a structured network that effectively holds drug particles in place, preventing sedimentation or caking. Its pseudoplastic behavior ensures the suspension exhibits high viscosity at rest. When subjected to shear force like shaking, pouring, or swallowing, its viscosity temporarily decreases, allowing for easy handling and administration.6 This is a critical property for patients requiring enteral feeding, as the shear-thinning behavior allows smooth administration through a narrow tube while the high viscosity at rest prevents settling within the bottle. The rheological control also enhances swallowability and mouthfeel by providing a smooth, consistent texture and preventing grittiness, which is particularly beneficial for patients with dysphagia.
Our formulation strategy incorporated additional excipients to further refine the suspension system. Glycerin acts as humectant, co-solvent, and stabilizing agent to prevent the formulation from drying out and impacting redispersibility. The system also includes tight pH control, which is essential for product quality, shelf life, and patient acceptability. In certain iterations, we also explored the strategic use of an ion exchange resin to further enhance taste masking capabilities, particularly for actives with more pronounced bitter taste. The result is a formulation that proficiently solved the challenge of bitterness and improved chemesthetic properties, such as throat catch or burning sensation associated with select poorly soluble NSAIDs.
This carefully balanced combination of MAS with rheology modifiers, humectants, and taste masking agents demonstrates our expertise in developing elegant and effective oral suspensions. Our innovative suspension system delivers improved dose uniformity, long-term physical stability, precise in-vitro dissolution, and better mouthfeel and swallowability, all of which contribute to superior patient compliance.
Beyond elevating overall product quality, it creates a visually appealing, dye-free formulation. Visual aspects should not be underestimated — patients often associate clarity, color, and consistency with product quality, and a suspension that looks elegant can inspire greater trust and acceptance.Overall, a comprehensive formulation approach reflects the reality that no single excipient, however novel, can solve every challenge. Success depends on understanding the interactions of multiple components working in sync.
A PATHWAY FOR FORMULATION EXCELLENCE
The successful journey from a novel excipient to an FDA-approved product is a compelling testament to the blend of expert formulation science and regulatory understanding. This case study demonstrates that excipients like MAS are powerful tools, but their full potential is unlocked through the strategic use of a complete excipient system. By successfully leveraging magnesium aluminometasilicate’s unique capabilities, this approach has shown that it is possible to create innovative, patient-centric suspensions that are both therapeutically effective and commercially viable. This experience highlights the critical role of expert formulation in a complex environment and offers a pathway for formulators seeking to accelerate their product development with new materials and methodologies.
REFERENCES
- Alqahtani, M.S., Kazi, M., Alsenaidy, M.A., et al. Advances in Oral Drug Delivery. Front Pharmacol. 2021 Feb 19;12:618411. doi: 10.3389/fphar.2021.618411. PMID: 33679401; PMCID: PMC7933596.
- Mittal, R.K., Zifan, A. Why so Many Patients With Dysphagia Have Normal Esophageal Function Testing. Gastro Hep Adv. 2024;3(1):109-121. doi: 10.1016/j.gastha.2023.08.021. Epub 2023 Oct 5. PMID: 38420259; PMCID: PMC10899865.
- Xie, B., Liu, Y., Li, X., et al. Solubilization techniques used for poorly water-soluble drugs. Acta Pharm Sin B. 2024;11:4683-4716. doi:10.1016/j.apsb.2024.08.027.
- U.S. Pharmacopeia. Quality Matters. Novel Excipients: New Hope for Therapeutic Innovations. Accessed August 7, 2025. https://qualitymatters.usp.org/novel-excipients-new-hope-therapeutic-innovations.
- Vanderbilt Minerals, LLC. VEEGUM® EZ Magnesium Aluminum Silicate. Accessed August 14, 2025. https://www.vanderbiltminerals.com/products/veegum-ez/rx-veegum-magnesium-aluminum-silicate.
- Layek, B. A Comprehensive Review of Xanthan Gum-Based Oral Drug Delivery Systems. Int J Mol Sci. 2024;25(18):10143. doi:10.3390/ijms251810143.

Mary Schuster earned her degree in biochemistry from the Georgia Institute of Technology in Atlanta, GA, and has built a career spanning nutraceuticals, molecular biology, and pharmaceuticals. She began her journey in the pharmaceutical industry as a scientist at Kiel Laboratories, where she gained hands-on experience in drug formulation and analytical method development. This foundational experience gave her a deep understanding of pharmaceutical operations and laid the groundwork for her current role as a Business and Innovation Strategist, where she bridges the gap between innovation and market-ready solutions.
Total Page Views: 3725













