Issue:November/December 2025
PLATFORM TECHNOLOGY - Developing Innovative Solutions to One of the Top Public Health Threats: Antibacterial Resistance
THE UNRESOLVED CHALLENGE IN TREATING BACTERIAL INFECTIONS
According to the World Health Organization 1.27 million people died in 2019 due to antibacterial resistance (AMR). AMR is commonly understood to be associated with infections which are no longer easily treatable by common antibiotics due to mechanisms of resistance in the bacteria. Additionally, elderly people, patients receiving antibody therapies, and oncology patients, amongst others are at additional risk for developing bacterial infections. Due to these complex host factors, these patients may not be effectively treated by traditional antibacterials, even when the bacteria is deemed to be susceptible. Infections are increasingly recognized as a major factor overturning the benefits of years of research, innovation and progress in modern medicine, as successful treatment for cancer, for example, may be negated by mortality due to infection.
Data shows that bacterial infections develop in ~40% of individuals with blood cancers, in over 50% of CAR-T cell therapy recipients, and 13% for patients who are on long-term corticosteroids. There is therefore a clear and urgent need for the development of novel approaches to treat infections in these underserved and vulnerable populations.
Taking oncology as an example, while immunotherapy provides new lifesaving options for treating and managing some cancers, the more established modalities of radiation and chemotherapy continue to play a critical and leading role in the overall treatment paradigm. Similarly, when we look at infections, traditional antibiotics are likely to remain the primary workhorses in infectious disease management even as novel modalities are developed and become available for the treatment of more complicated cases. However, as with oncology, there will be patients and cases in infectious disease where immunotherapy transforms outcomes. Immunotherapy, therefore, holds the potential to revolutionize the treatment of ‘hard to treat’, chronic, and often recalcitrant infections.
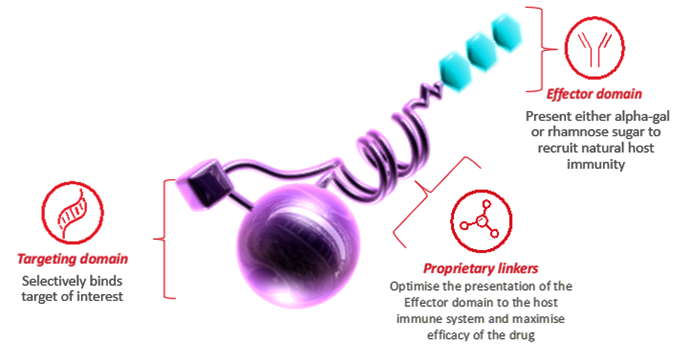
NOVEL IMMUNE THERAPY APPROACH FOR INFECTIOUS DISEASES – HARNESSING ALPHA-GAL
A robust antibody response to alpha-gal was detected back in the 1960s in the context of xenotransplantation, when the transplanted tissue was rapidly attacked and killed due to the high-density presentation of alpha-gal sugar on the surface of the pig tissue being recognized by abundant anti-alpha-gal antibodies present in the human. Because all mammals (except old-world primates) present this alpha-gal sugar epitope and humans do not, humans have developed antibodies to this sugar by way of regular exposure in the gut. Following the identification of the alpha-gal antibodies, additional research identified other anti-sugar antibodies, such as rhamnose antibodies with similar properties to alpha-gal. These very abundant polyclonal antibodies are low affinity but have high avidity, and are thus recruited within the context of a high-density glycan array.
The anti-sugar antibodies are abundant polyclonal antibodies, including IgM and IgG isotypes. These multiple isotypes leverage different aspects of the immune system and drive multiple pathways of immune engagement. Multiple research teams have been aware of the opportunity to take advantage of these ubiquitous pre-existing anti-sugar antibody repertoires, and there has been much excitement about how to take advantage of these antibodies to develop novel immunotherapeutics in oncology and infectious disease, or to enhance the efficacy of vaccines.
The Alphamer® platform takes advantage of this opportunity. Based on a novel and proprietary linker technology that allows for the non-cleavable coupling of a targeting moiety to a sugar effector moiety, this rational coupling allows for the successful presentation of the selected sugar to the immune system, while remaining bound to the target cell via the targeting moiety. This binding of the molecule to the target cell while maintaining presentation of the sugar to the immune system allows for rapid immune-driven killing of the target cell.
THE TRANSLATIONAL CHALLENGE
The fundamental aim of any anti-infective is to reduce the pathogen load sufficiently to enable the patient’s immune system to clear the rest of the infection. In individuals with a less robust immune response, including the elderly and those with compromised immune systems, the anti-infective is often doing its job in terms of reducing pathogen load, but the patient isn’t actually improving. This is because the immune system of the individual is unable to fully eradicate the pathogen, which may lead to chronic infections, or infections which go on to develop resistance due to significant and continued drug pressure.
One reason why antibiotic development has historically been attractive for investors and pharmaceutical companies alike is that, for traditional antibiotics, animal models of infection and other pre-clinical activities translate quite well to efficacy in otherwise healthy individuals. Identifying the optimal drug exposure, which leads to bacterial clearance in the chosen animal models and then extrapolating that exposure into humans, within defined safety margins, saw a lot of early success. However, as noted above, these traditional antibiotics may have limitations when it comes to their efficacy in individuals who have some type of comorbidity, and the animal models themselves are limited in terms of what they can tell us about efficacy in hosts with varying immune status.
PRE-CLINICAL MODELS FOR NON-TRADITIONAL ANTI-INFECTIVE MOLECULES
The Alphamer platform has enabled development of a bifunctional molecule that has both direct acting antibacterial activity as well as robust and rapid activity driven by the immune recruiting component. Because of this novel mechanism of action, new techniques are required to assess the potency and activity of the molecules, replacing the traditional, and often inadequate, animal models of infection, to capture the effect of the molecule in totality.
As previously mentioned, the vast majority of pre-clinical mammalian models have either no or very reduced anti-sugar antibodies, which means the anti-sugar antibodies must be administered as part of any model of infection in such a way to achieve the human equivalent titer. Additionally, mouse models typically used for infection studies are often neutropenic, which of course limits the ability to evaluate the role of the immune system in bacterial clearance. Finally, complement is a major player in the activity of immune recruiting molecules, but complement in mice is known to be quite different from that in humans, with levels only around 10% of those in humans.
The challenges associated with the differences in the immune systems of mice and humans are just one aspect that needs to be addressed for the evaluation of novel non-traditional molecules. It is encouraging that multiple research groups in the industry are publishing on the use of novel assays for the evaluation of anti-infectives and are working to address some of the limitations of more traditional animal models and assays. Expanding the use of more host-relevant conditions, including a whole blood assay, which has commonly been used in immunology, inflammation, and hematology, is a great starting point. Being able to demonstrate the totality of the effect of novel molecules in a relevant human assay, coupled to the data achievable in less than perfect animal models, is an exciting success.
THE NEED FOR ONGOING EDUCATION BEYOND THE CLINIC
In addition to either developing or adapting novel assays for evaluation of immune therapies to treat bacterial infections, additional pragmatic challenges also need to be addressed.
Traditional antibacterials have, in part, their suitability for clinical use evaluated using standard and widely used susceptibility tests as noted above. These tests perform reliably and effectively for traditional antibiotics. How to adapt these susceptibility assays is a key question under discussion, whether for novel immunotherapeutics such as Alphamers, or for monoclonal antibodies or phage therapies. As the standard assays are modified to ensure utility and new technologies or tests are developed, significant education and adoption of these technologies will be required to reassure clinicians that the right drugs are being used in the right patients at the right time.
A serious risk is that powerful and important new therapeutic modalities will be undervalued or underused while efforts to understand their susceptibility lag behind. Those developing non-traditional agents must work in close collaboration with subject matter experts, funders and regulatory authorities to facilitate collective advancement in the field. Both the adaptation of current methods and the development of new approaches to minimum inhibitory concentration (MIC) testing will enable healthcare providers to confidently use drugs with novel mechanisms of action. This will be important for most, if not all, of the novel technologies working their way through development, as the traditional assays and workstreams in the microbiology lab are not well suited to emerging modalities.
SUMMARY
Antibacterial resistance remains a critical and unresolved challenge, particularly for vulnerable patient populations. Realizing the potential of emerging therapeutics, particularly immunotherapeutics, requires addressing key translational challenges, such as developing more relevant preclinical models and adapting susceptibility assays. Tackling these challenges will help ensure that innovative anti-infective strategies reach the patients who need them most, improving the management of hard-to-treat infections and addressing a major public health threat.

Dr. Jennifer Schneider is CEO of Centauri Therapeutics. Prior, she was executive officer of North America for the Global Antibiotic Research & Development Partnership (GARDP). She has nearly 20 years of experience working with companies, universities and non-governmental organizations in the United States and around the world, providing expertise in antibiotic policy, partnering, and fundraising. She participates in US and international discussions surrounding market creation, access, and novel payment models. She earned her PhD in Molecular Biology from the University of Notre Dame and her MPH in Epidemiology and International Health from the University of Michigan.
Total Page Views: 1671













