Issue:October 2017
TABLET FORMULATION - Reformulation of Tablets to Resolve Sticking & Picking Issues Faced on Compression: A Case Study
PURPOSE
In this study, Metrics Contract Services was tasked with a complex re-formulation project. Briefly, the free-acid form of an API, Compound XY, was previously manufactured as 5-mg, 10-mg, and 25-mg strength tablets using direct compression process. But the free-acid form of the compound proved to be poorly soluble in aqueous media. Hence, to increase the solubility of Compound XY, a salt form of the drug was explored.1 Out of all the salt forms investigated, potassium-salt was found to be the least sticky and least hygroscopic; therefore, it was selected for further study.
Metrics scientists were tasked with manufacturing the potassium-salt form tablets of Compound XY at all of the above strengths using the same tablet composition used to produce the free-acid form tablets. While manufacturing the potassium-salt form tablets, Metrics scientists observed various problematic issues described further. The blend flow from the hopper to die cavity was very poor, resulting in undesired weight variation. Additionally, the tablet surface showed discoloration, an effect that worsened with increasing tablet strength. As a result of the study presented here, the potassium-salt form of Compound XY was reformulated to yield tablets of acceptable dissolution, weight, properties, and appearance without adding any new excipients to the formulation.
BACKGROUND
The tablet weight of both the 5-mg and 10-mg formulations was 64 mg; the tablet weight of the 25-mg formulation was 160 mg. In order to expedite the reformulation development, it was decided to follow the same formulation process originally used to manufacture the free-form of Compound XY. Diverting from the previous formulation process would have affected the analytical methods.
During the manufacturing of 10-mg strength of Compound XY, scientists observed weight variation, so the tablet press was adjusted to accommodate the tablet weight. At this point, discoloration was observed on the tablet surface. Hence, the tablet press was disassembled, and the feed frame was inspected. It was discovered that the powder blend itself was discoloring as a part of the compression event. Additionally, the blend was sticking to the feed frame and the die table. Figure 1A shows the sticking of the blend to the die table and the scrapper.
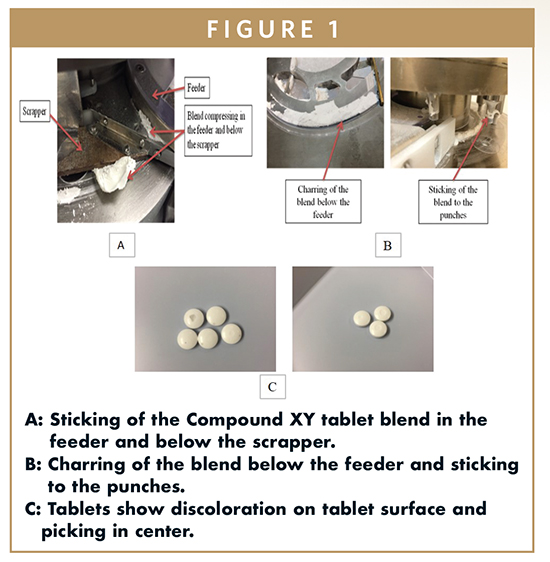
To avoid discoloration and resulting tablet weight variation, scientists stopped the tablet press every 5 minutes to remove and clean the scrapper. They conducted an all-process test to inspect issues such as hardness, thickness, and disintegration; everything met project specifications. Hence, compression was continued and yielded tablets with acceptable properties.
Similar compression issues were seen while manufacturing the 25-mg strength tablets of Compound XY. Indeed, due to the fact that the 25-mg strength contained the highest amount of API, those tablets delivered the worst results in terms of surface discoloration, sticking, picking, and chipping. The blend was sticking beneath the feeding and getting charred. It is seen in Figure 1B. The blend also was sticking to upper and lower tooling.
The tablet surface showed discoloration and picking in the center of the tablet, shown in Figure 1C. Scientists observed that some of the tablets were capped as they came off the press, but no capping was seen in friability. In order to circumvent these issues, scientists compressed the blend at a very slow speed of 5 rpm. Despite reducing speed, the same issues were observed. Scientists thus concluded that the compression problems were caused not by the compression process but, rather, by the inherently sticky nature of the blend. In order to resolve this issue, scientists studied the formulation composition of these tablets to identify the root causes of the stickiness.
A direct compression process had been employed to manufacture tablets of Compound XY. Direct compression is a well-known and popular choice for manufacturing tablets given its speed and efficiency and because it is the least complex way to manufacture tablets. In this process, the API was blended with excipients and lubricated and then followed by compression. A wide variety of APIs that are heat and moisture-sensitive can be used in this type of process. However, one must be cautious when selecting excipients during this process compared to other processes, such as granulation. Excipients used in this process must demonstrate good flowability and compression for successful operation.2
The original formulation of the tablets contained silicified microcrystalline cellulose (Prosolv SMCC 90), mannitol, and Avicel HFE as inactive excipients. They were primarily used as a diluent; crospovidone was used as a disintegrating agent, and magnesium stearate was used as lubricant. The drug loading was 17 w/w in the original formulation.
Silicified microcrystalline cellulose is a pre-mixed blend of colloidal silicon dioxide and microcrystalline cellulose. Avicel HFE is a mixture of mannitol and microcrystalline cellulose; it contains 8% to 12% mannitol. In all, this formulation was made up of more than 45% mannitol.
Mannitol is known within the pharmaceutical industry to cause stickiness during compression, a problem exacerbated by the potassium-salt form of Compound XY, which is sticky in nature.3
Also, formulations containing mannitol require lubricants because they are less flowable. The study conducted by Hutchins et al shows that as the percentage of lubricant in mannitol-containing formulation is increased, the sticking of the powder blend to the punch surface decreases.3 Hence, it was concluded that mannitol had a relatively high affinity for the metal punch surface, which is reduced by addition of the lubricant.
The effect of mannitol grade on sticking was also evaluated. Powdered mannitol shows higher sticking propensity as compared to granular mannitol. It was shown that diluting the mannitol blend with microcrystalline cellulose and 1% magnesium stearate decreased the sticking propensity of the blend. Based on the literature, it was concluded that to resolve the sticking and flowability issue, scientists needed to replace or reduce the amount of mannitol from the original formulation of Compound XY.
Inactive excipients can be removed from formulations without adversely affecting analytical methods. It is when scientists add new inactive excipients that they could detrimentally affect analytical methods for the product or at the least would cause methods to be re-evaluated.
METHOD
The direct compression process was used to formulate tablets of Compound XY using common blend approach. Trials consisted of screening three commonly used diluents: Prosolv SMCC90, Avicel PH102, and Avicel PH200. The percentages of excipients crospovidone and magnesium stearate used in the original formulation were kept the same in all reformulations. Powder properties such as bulk density, tap density, and Flodex were studied. Selected formulations were scaled up, and tablets were compressed using rotary tablet press. Tablet physical properties, such as friability, thickness, and disintegration, were studied, and a stability study was conducted. Tablets were packaged in 30-count in 60 cc bottles fitted with 33-mm cap with induction seal for stability study at 40°C and 75% RH, an industry standard.
RESULTS & DISCUSSION
In order to replace or reduce the amount of mannitol, scientists conducted several formulation trials to identify appropriate diluents. The goal was to use direct compression process and have the blend show good flowability because this was expected to resolve the challenge of weight variation. Inactive excipients, such as Prosolv SMCC90, Avicel PH102, and Avicel PH200, were used in order to investigate improving the flow property of the blend.
A common blend approach was chosen to manufacture 5-mg, 10-mg, and 25-mg tablets. In order to use common blend approach, tablet weight of the 25-mg formulation was increased to 300 mg. The percentage of crospovidone and magnesium stearate was kept the same. The tablet blends were manufactured using diluents like Prosolv SMCC 90, Avicel PH102, Avicel PH200, and a combination of Prosolv SMCC 90 and mannitol.
The flow property of the blend was measured using the Flodex powder analyzer. The Flodex of the Prosolv SMCC 90 blend was found to be 16 mm; the Flodex of Avicel PH 102 blend was 20 mm; the Avicel PH 200 blend did not flow in Flodex, and the Flodex of Prosolv SMCC 90 and mannitol blend was found to be 20 mm.
Out of all diluents studied, the blend manufactured using Prosolv SMCC 90 had the best flow compared to other diluents. To improve flow further, scientists added 1% colloidal silicon dioxide to the blend. Addition of 1% colloidal silicon dioxide improved the flow property to 12-mm Flodex. The reformulated tablets had lower drug loading as compared to original formulation, and the percentage of Prosolv SMCC 90 was increased from 25.94% to 84.36% in the reformulated tablets. All percentages of all other excipients like crospovidone and magnesium stearate were kept the same. Compression of this blend showed acceptable physical and dissolution properties with no discoloration on tablet surface and no sticking or picking.
A stability study of original formulation and reformulation batches of Compound XY was carried out in 40°C/75% RH. The 3-month and 6-month stability samples of the original formulation of Compound XY revealed tan spots on the tablet surface. In addition, the 6-month analysis of Compound XY tablets, 25 mg, revealed a split tablet. These issues were also observed during compression of Compound XY Tablets, 25 mg. The stress relaxation in the tablets was one of the probable causes of the tablets splitting during stability study.
The dissolution profile of the original Compound XY tablets, 25 mg, and reformulated tablets of Compound XY, 25 mg, and its stability samples is shown in Figure 2A and 2B, respectively. Scientists observed that accelerated stability conditions had no effect on the dissolution profiles of either the original or reformulated batches of Compound XY tablets, 25 mg.
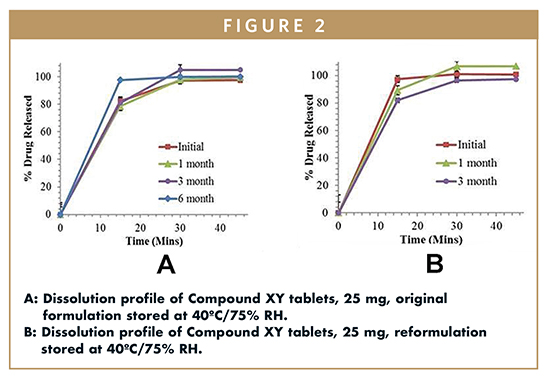
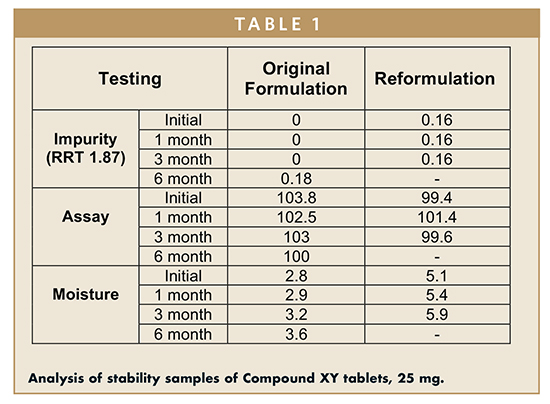
Table 1 shows the analysis and impurity profiles on the stability samples of the Compound XY tablets, 25 mg. The original formulation shows the impurity at RRT 1.87 at the 6-month time point, whereas the reformulated batch shows the impurity at RRT 1.87 in initial testing which remained constant over time.
CONCLUSION
Metrics scientists successfully reformulated tablets using the potassium-salt form of Compound XY without adding any new excipient. Through the reformulation, scientists also resolved earlier issues faced during tablet compression, namely sticking, picking, poor flow, weight variation, and discoloration.
REFERENCES
1. Serajuddin Abu TM. Salt formation to improve drug solubility. Advanced Drug Delivery Reviews. 2007;59(7):603-616.
2. Shangraw RF. Compressed tablets by direct compression. Pharmaceutical Dosage Forms: Tablets 1.1989:195-246.
3. Mullarney MP, Bruce MC, Hutchins A. Assessing tablet-sticking propensity. Pharmaceutical Technology. 2012;36(1):57-62.
To view this issue and all back issues online, please visit www.drug-dev.com.
B I O G R A P H I E

Dr. Smruti P. Chaudhari is Development Scientist I at Metrics Contract Services, where she is responsible for preformulation, formulation, and process development, and clinical manufacturing for projects from pre-IND phase through Phase III. She also manages scale-up and technology transfers for solid oral dosage forms. She earned her PhD in Pharmaceutics and Drug Design from Long Island University, from which she previously earned her MSc in Industrial Pharmacy.

Dr. Anshul Gupte manages all aspects of operations related to formulation and manufacturing of a client’s clinical trial materials. As Associate Director, he supervises a team of formulation scientists, specialists, and technicians who develop solid dose formulations for Phase I-III clinical trials — and who scale-up and validate those materials as needed. He oversees clinical trial batch manufacturing and packaging under cGMP guidelines, supervises technology transfers, and documents clinical trial material batch records.
Total Page Views: 12508

















