Issue:October 2015
PRIMARY PACKAGING - Ophthalmic Squeeze Dispenser (OSD): Does One Size Fit All?
INTRODUCTION
The discussion about the use of preservatives in eye drops is still controversial, but more and more evidence supports the use of unpreserved eye drops for treatment of chronic diseases. For example, dry eye, a condition in which the tear film is impaired, is increasingly more recognized as an inflammatory disease. Treatment with preserved eye drops may worsen symptoms. For the treatment of mild symptoms, the use of non-irritating or unpreserved artificial tears is recommended, while as for more severe cases, the use of an anti-inflammatory principle will become the standard treatment. The same recommendation for restricted use of preservatives applies for glaucoma, which requires a treatment for the rest of life. Baudoine et al have shown that the risks for glaucoma patients to experience severe local side effects increases with the number of preserved eye medications taken in parallel.1 Combination products that reduce the number of necessary medications or the use of unpreserved eye drops will certainly help suffering patients and will also increase adherence to the prescribed treatment schedule. At least in Europe, the authorities have recognized the issue, and the European Medical Agency (EMA) pushes, for example, the use of unpreserved ophthalmic formulations, most recently in a guideline on pediatric medicine.2
Recognizing the trend toward preservative-free topical drugs, in 2006, Aptar Pharma started the development of the Ophthalmic Squeeze Dispenser (OSD), a multi-dose device designed for unpreserved eye drops.3 The system is designed in a common squeeze bottle shape to ensure user acceptance. The first commercial product registered under the European Medical Device Directive using the OSD as primary packaging was VISMED Multi, an artificial tear product by Swiss Eye Care expert TRB Chemedica, introduced to the market in 2011. To date, more than 25 million OSDs were sold as preservative-free multi-dose systems for several products, and many new projects are on the way. The key question we will answer in this review is how a single device can fit a wide range of formulations for different conditions and diseases?
WHERE ARE THE CHALLENGES?
Eye drops can be very different from each other, and their behavior is very difficult to predict. Artificial tears used to supplement the tear film do not contain active ingredients but vary in rheological properties, such as viscosity, depending on patients need from low to highly viscous. Eye drops containing active drugs, eg, glaucoma treatment, may contain a lot of auxiliary compounds to stabilize the formulation, and the content of active pharmaceutical ingredient may range from micrograms to few milligrams per milliliter. The dropper should always deliver the appropriate drop size, the formulation must be stable for storage and in-use period, and the forces to deliver a drop should remain in a reasonable range. What needs to be considered when putting a formulation into the OSD?
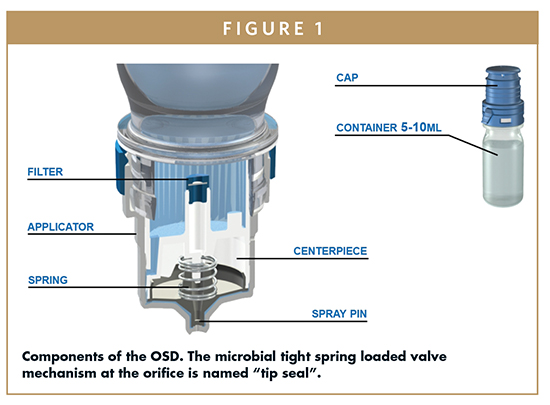
HOW THE OSD WORKS
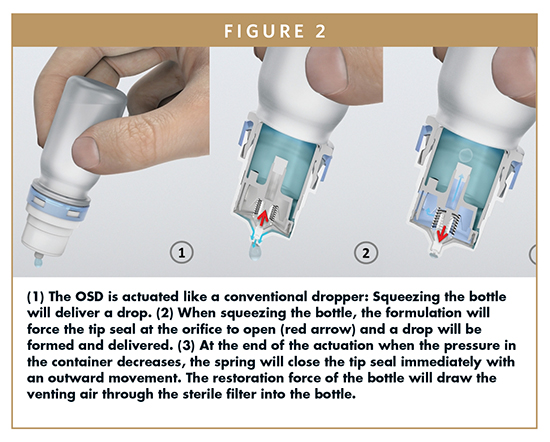
To understand the system, its flexibility, as well as technical limits, it is useful to explain how it works. The system follows a pure mechanical approach to prevent microbial contamination of the product and uses the so-called “tip seal technology,” a specifically developed outlet valve technology, and sterile filtration of the venting air. Like any other conventional dropper bottle, the OSD needs to be held in an angular or complete upside down position to deliver drops. The system is primed by squeezing the bottle until the first drop is delivered.
During the actuation process (squeezing the bottle), the pressure within the system rises, and the liquid is forced through the liquid channel into the space between spray pin and applicator. As soon as the liquid pressure exceeds the valve spring force of the tip seal, the central part of the spray pin is forced backward, and the orifice opens and liquid is dispensed. The special outer geometry of the orifice allows the product to form and deliver a drop. The tip seal outlet valve closes immediately with an outward movement when the pressure drops below a defined threshold. This function is self-regulating and prevents any backflow into the system and thus potential microbial contamination. During the actuation in angled or upside down position, the bottle content will submerse the filter. The side of the filter membrane that faces the formulation is highly hydrophobic, which prevents wetting of the membrane or that some part of the formulation can be forced through the filter. Dispensing drops reduces the volume inside the container; consequently, an equilibration of the container content is required. The restoring force of the bottle, which is created by returning to its initial shape, equilibrates the pressure difference by drawing air into the container through the filter, preventing microbial contamination of the formulation via the venting air. It should be recognized that the flow path for the formulation and that for the venting air are completely separated. This venting function is independent from device orientation.
HOW TO ENSURE A SMOOTH ACTUATION?
When squeezing the bottle, the pressure will force the formulation trough a specific fluid pathway into the space behind the spray tip, which subsequently opens the tip seal outlet valve. For a smooth actuation, the diameter and length of the flow channel between bottle and space next to the orifice are important. The length of this flow channel can be varied, thus the resistance of the system adapted to the viscosity of the formulation. The length of the internal channel can be set in multiple steps to get the best results for a given formulation. This feature is referred to as flow control. The optimum flow control for each formulation is determined by experiments in the lab using completed systems with different preset flow control values.
ACTUATION FORCE
Actuation force for conventional droppers for preserved formulations with open orifice depends mainly on the stiffness of the bottle, which is determined by wall thickness and the used material (eg, softer polyethylene or stiffer polypropylene). Compared to the open-bottle designs, the actuation force of the OSD is influenced by three factors: tip seal function, liquid level of the bottle, and stiffness of the bottle. As previously described, a certain pressure needs to be generated inside the system to open the tip seal. In a first-generation, the actuation force was quite high in order to provide a real tight sealing of the orifice. Continuous improvement and product optimization have significantly lowered the force to actuate and as such, improved patient compliance and convenience.
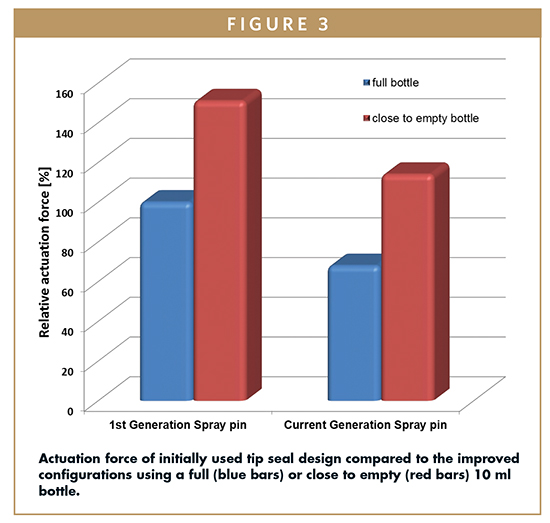
An important factor influencing the actuation force is the liquid level within the bottle. A full bottle requires less squeeze force to deliver a drop than one which is close to being empty. In an almost empty bottle, more air must be compressed before the opening pressure of the outlet valve is reached. Therefore, it is recommended to use a bottle size close to the intended filling volume. Starting from the standard 10-ml LDPE (low density polyethylene) bottle, an oval-shaped (5-ml) as well as a round 7.5-ml bottle (Figure 4) is now available to meet the requirements of the market.
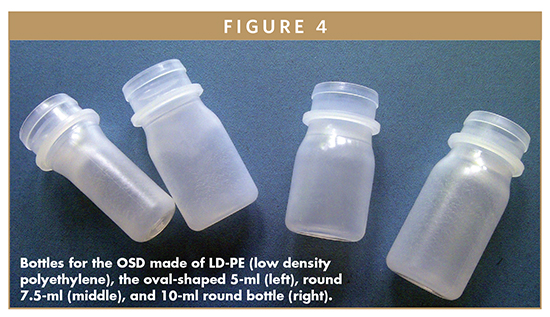
The bottles for the OSD are made from soft polyethylene. The wall thickness was optimized to balance between low actuation force, managing water vapor transmission rates and maintaining sufficient restoring force to enable ventilation of the system.
COMPATIBILITY WITH FORMULATION
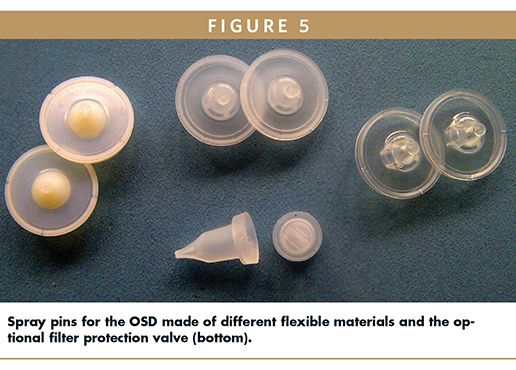
When handling liquids in delivery devices, there is always some potential risk of interactions which may impair the stability of the formulation or function of the entire systems. Any such potential compatibility issues should be considered early in development and tests addressing such issue are mandatory. The highest potential risk is sorption of active ingredients to material of the container closure system. In addition to the mentioned use of polyethylene and polypropylene, a thermoplastic elastomer (TPE) material is used for the outlet valve. This part is named spray pin (Figure 5), which comes in contact with the formulation. Aptar identified different elastomeric materials that may be used in cases when the standard material is creating compatibility concerns.
As previously described, when using the OSD, the formulation will submerse the filter. Its hydrophobic membrane will prevent formulation from impairing its proper function. This is true for most formulations. But there are also formulations known for a very low surface tension, which allows wetting the membrane or even ingress into the filter pores. If this happens, normally the venting of the system is impaired, and the restoring force of the bottle is not sufficient to bring it back into its initial shape. As a result, the required squeeze force will increase step by step for the subsequent actuations. The technical solution for such formulations is the use of a so called filter protection valve (Figure 5, bottom), a small component made of TPE resin that is slipped over the filter. This measure prevents the formulation coming into contact with the filter membrane without affecting the venting of the system.
Another topic related to the bottle is its sterilization procedure. The standard procedure for sterilization of the low-density polyethylene bottles is gamma-radiation with more than 25 kGy. According to the literature, radiation sterilization may have an effect on the exposed material, although polyethylene is well suited for this procedure. Ethylene oxide (EtO) sterilization may be used as an alternative for the bottle.
Because of its complex design, the OSD dropper part needs to be radiation sterilized. For the OSD, a validated gamma sterilization process according ISO 11137-1 and 2 was established.
CRYSTALLIZATION AROUND THE ORIFICE
As previously described, at the end of the actuation, the tip seal valve closes with an outward movement, leaving some formulation visible at the orifice. This remaining drop (typically 3 to 7 microliters) will dry out within the next 4 to 6 hours even when the protection cap is re-attached. For some formulations, the remaining material from these liquid bears the risk of blocking the orifice due to crystallization after a few days of use. This potential risk can be tackled by introducing a porous liner material into the protection cap. When the protection cap is re-attached onto the OSD after use, the liner material will take up the remaining drop and spread the liquid within the material on a large surface. This will speed up drying and thus further reduce potential growth of microbes, preventing crystallization around the orifice. If this technical solution is suited for a particular formulation, it must be tested carefully. The impact of a potential uptake of the drop as well as the drying rate has to be understood; also the intended number of doses per day needs to be taken into consideration.

SUMMARY
The wider use of preservative-free multi-dose devices for eye drops has started in Europe for artificial tears. Patients and consumers appreciate the convenient and intuitive handling of the OSD, properties which are important for the further success of this device. For pharmaceutical manufacturers, the cost effectiveness of OSD is another important aspect. The system is flexible to meet the challenge of different ophthalmic formulations. However, professional guidance is needed and provided by Aptar Pharma to find the optimum configuration. The provided technical measures were thoroughly tested with special emphasis on the mechanisms, ensuring microbial integrity of the system before the OSD was introduced to the market. Validated 100% in-process controls (IPCs) during manufacturing of the OSD ensure the reliability of the system. Although this constitutes a thorough validation of the system itself, it cannot exempt marketers from performing proper due diligence on the finished product. But the qualification work performed by Aptar Pharma helps smooth this process significantly and allows for shorter time to market. Special attention was always paid to ensure safety, obviously with special emphasis on microbial integrity of the system.
REFERENCES
1. Baudouin C, et al. Prevalence and risk factors for ocular surface disease among patients treated over the long term for glaucoma or ocular hypertension. Eur J Ophthalmol. Epub June 11, 2012.
2. Guideline on pharmaceutical development of medicines for paediatric use, August 2013, EMA/CHMP/QWP/805880/2012 Rev. 2.
3. Marx D, Birkhoff M. New devices for dispensing ophthalmic treatments may be the key to managing the life cycles of established products. Drug Development & Delivery. 2010;10(9):16-21.
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Degenhard Marx, following the study of Veterinary Medicine and the successful completion of his thesis at the University of Leipzig, joined the Arzneimittelwerke Dresden/Asta Medica cooperate research in 1992. In 2001, he took over a Senior Research position at Altana Pharma/Nycomed in Constance, Germany. During this time in the pharmaceutical industry, he collected ample experiences in the drug development of anti-inflammatory and cardiovascular drugs. In 2008, he became Business Development Manager at Ing. E. Pfeiffer, Pharma Division, which became Aptar Pharma in 2010. He is now Director of Scientific Affairs within the Aptar Pharma Consumer Health Care division.

Matthias Birkhoff is Vice President, Marketing, of Aptar Pharma CHC Division. In this role, he is responsible for Aptar Pharma’s Eye Care program and coordinates research and development activities, microbiological assessment, and commercial strategies. Mr. Birkhoff started his career in pharmaceutical sales in a major multinational pharmaco before joining Aptar 16 years ago. Before getting involved in Business Development and Marketing, he was in charge of sales in the AsiaPacific region. He studied medicine at the University of Dusseldorf, Germany, and holds a nursing degree.
Total Page Views: 10104

















