Issue:October 2013
INTRADERMAL DELIVERY - Advances in Intradermal Drug Delivery
INTRODUCTION
Vaccines have helped reduce the instance of infectious diseases around the globe, nearly eradicating past plagues, such as smallpox and polio, and reducing preventable infections to the point where few people experience the effects of measles, pertussis, and other illnesses.1 While no vaccine is 100% effective, the incidence of infectious diseases continues to decline. Although a variety of options for vaccine delivery exist, including intramuscular (IM), subcutaneous (SC), nasal, oral, and transcutaneous methods, recent advances have helped improve the efficacy of vaccine delivery through intradermal (ID) injection.
Currently, IM or SC injection through the use of needle and syringe is the most common method of vaccine delivery, with many of the vaccines on the market using this mode of delivery. However, the skin contains a high concentration of antigen-presenting cells, making it an ideal location for injection. These cells perform an essential role in processing incoming antigens, resulting in powerful immune system responses. Delivery of vaccines to the epidermis or dermis may result in superior immune responses when compared to IM or SC injections.2 In addition to the enhanced immune response in patients, ID delivery offers a variety of benefits to pharmaceutical manufacturers, including dose sparing, increased availability of limited or expensive antigens, and reduced cost per dose.
Unfortunately, the current method of ID delivery can be difficult to master. ID injection is typically administered using the Mantoux technique, which requires special training and may not effectively target the skin, resulting in delivery to the SC tissue or leakage. Developed by Charles Mantoux in the early 20th century, this method requires that healthcare practitioners master inserting a needle at a 5 to 15 degree angle approximately 1 mm deep into the skin to inject the vaccine effectively.2 When the injection is performed correctly, a bleb or wheal of 6 to 10 mm in diameter is formed on the skin, indicating the vaccine has been delivered to the dermal space. The bleb goes away after a few minutes as the vaccine is dispersed and absorbed into the surrounding tissue. The difficulty associated with training and the inconsistency of injection efficacy have deterred medical practitioners from using ID injection as a common immunization method.
Alternative methods of ID administration are now being studied to help provide the market with a simple, reliable method of ID injection. Novel delivery devices may aid needle/syringe injection, while jet injection, transdermal patches, and micro-needles offer new options to patients and caregivers. Clinical studies have been conducted on ID delivery for a variety of illnesses, including influenza, hepatitis B, hepatitis A, polio, measles, yellow fever, and more.2 There are several therapeutic cancer vaccines using dendritic cells delivered by ID injection. Intradermal delivery of DNA vaccines combined with electrophoresis have been shown to enhance delivery.3 For researchers conducting clinical trials using Mantoux ID injection, there is the additional question of whether the lack of efficacy is due to the vaccine or the injection technique. The advantages of ID injection, coupled with advances in ID administration technology, can help make this method a more desirable route of administration for future vaccines.
A DIFFICULT ROAD: CAN ADVANTAGES OF ID INJECTION OUTWEIGH DIFFICULTY OF DELIVERY?
Although there are more than 95 clinical studies using ID injection as a delivery method currently underway, barriers still exist to the use of ID injection.4 While the US, Europe, and other developed countries have private insurance and/or government funding in place for vaccination and rely on single-dose formats, emerging markets tend to favor multi-dose formats and are limited by cost and availability of supply. In addition, most vaccines on the market today require cold chain storage and distribution, placing an added burden on limited resources in the emerging markets or the limited refrigerator space at the healthcare provider. Loss of efficacy can result if the vaccine is not maintained at 4°C.
Clinical trials are tasked with optimizing the ID dose by comparing different size doses (antigen and volume) and demonstrating that the immune response is comparable to IM or SC injections. Regulatory considerations must also be taken into account, particularly if a pharmaceutical manufacturer needs to develop a new formulation, primary package, or device for the vaccine formulation.
However, the benefits of ID injection may outweigh other considerations. Not only does ID injection offer pharmaceutical companies a way to differentiate their product, it also provides an opportunity to reduce the cost of each dose through dose sparing. With ID injection, a reduced dose may be able to produce the same immune response as from a comparable IM/SC dose. This dose sparing results in reduced cost per dose and a reduced volume of the vaccine in the cold chain. Such a reduction can help to increase the amount of antigen available, making it more accessible in the global market, while reducing the cost associated with cold chain storage needs.
Additionally, ID injection may help improve vaccine efficacy in hard-to-treat populations, including the elderly (due to immunosenesence) and infant populations (due to immature immune systems), which typically cannot receive standard vaccines.4,5 ID injection in these populations can help overcome low immunogenicity to standard vaccine dosages and routes of administration.
DELIVERY SOLUTIONS
More than 200 years ago, vaccines were delivered by creating small holes in the skin.2 However, poorly controlled dosing, sterility concerns, and inefficient use of the vaccine had the industry seeking a new solution. Today, a variety of options for ID delivery exist in addition to the most popular method of needle injection using the Mantoux method. Options include syringe-based micro-needles; patch injections; coated, hollow, or dissolving micro-needles; and jet injectors, as well as delivery devices, such as adapters that can be used to aid ID delivery. Each of these methods offers pros and cons when it comes to ID delivery.
Use of a needle or syringe requires no specialized equipment, but training in the Mantoux method can be difficult. Patch injection devices do not use a conventional needle, so the injection itself may be less invasive and painful, as well as easier to administer, for patients. However, this method may require a reformulation of current vaccines, which may be time consuming for pharmaceutical manufacturers requiring process development and new regulatory submission.
Jet injection provides a needle-free technology that can eliminate reuse of needles and cross-contamination if auto-disable cartridges are used. Unfortunately, jet injection devices can be expensive, and require a substantial capital investment. Not all markets will have the resources to purchase this type of device. Additionally, there will still be pain on injection for the patient as the liquid is pushed into the skin at high pressure.
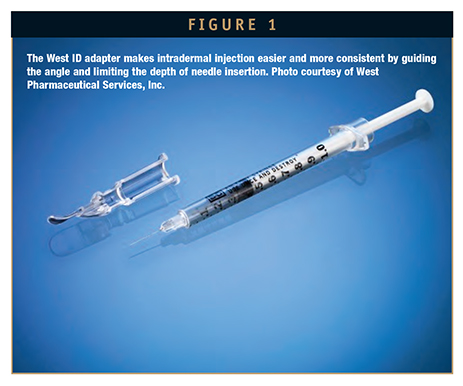
Adapters, including the ID Adapter from West, can provide a guide for injection when using a syringe system. The device fits over a conventional hypodermic needle and syringe and precisely controls the angle and depth of needle penetration into the ID layer. The ID adapter offers healthcare professionals a simple way to perform an ID injection, without the need to reformulate their vaccine. Use of an adapter can offer greater confidence that the healthcare provider has administered the vaccine into the correct space.
RECORDS OF SUCCESS: ID USE FOR RABIES VACCINES
Around the world, more than 17.4 million people are exposed to rabies through animal bites each year. Occurring in more than 150 countries and territories, rabies is a viral disease that can cause encephalitis when transmitted to humans. If not treated, rabies is 100% fatal, and has caused more than 55,000 deaths annually, with the highest incidences occurring in Africa and Asia.6
Traditional vaccinations for this deadly disease include a pre-exposure vaccination that consists of three full intramuscular doses of cell-culture or embryonated-egg-based vaccine. Dose size is either 1 mL or 0.5 mL based on the type of vaccine used. The doses must be given 7 days apart, although minor variations in the timing will not prohibit immunization. Modern rabies vaccines are well tolerated, but cost can be a factor in determining whether or not a patient receives pre-exposure vaccination. To help reduce the cost of cell-derived vaccines for pre-exposure rabies vaccination, ID vaccinations can be used. The administration regimen consists of 4 doses of 0.1 mL over the course of 28 days.7 While the ability to administer the dose requires more specialized training, the cost savings may encourage greater use of the pre-exposure vaccination.
The Intradermal Rabies Vaccine (IDRV) was approved for use in developing countries by the World Health Organization (WHO) in 1992. Often, developing countries face issues of vaccine shortage and lack of funding, and the cost of post-exposure prophylaxis (PEP) can be prohibitive. Intradermal regimens for rabies post exposure, the Thai Red Cross two-site regimen and the eight-site regimen, are currently recommended by the WHO. However, a four-site regimen has been evaluated that reduced the cost of PEP by more than 60%, when compared with the standard IM injection regimen (Essen). The four-site regimen also reduced the number of patient visits required when compared to the Thai Red Cross method, and offers convenience over the eight-site regimen. These ID regimens, which were first endorsed by WHO’s Strategic Advisory Group of Experts on Immunization in 2007, offer a cost-effective alternative to traditional intramuscular administration while providing effective and safe immunization.7
For post-exposure prophylaxis, IM vaccination should consist of either a five-dose (the Essen regimen) or four-dose regimen. Intradermal administration of a cell-culture and/or embryonated-egg-based vaccine has been successfully used in a much reduced dose: 0.1 mL for purified Vero cell rabies vaccine and 0.1 mL for purified chick embryo rabies vaccine.8
In addition, a study conducted by the Chinese University of Hong Kong has found that ID administration of human papillomavirus (HPV) vaccines may be dose-sparing and cost-saving. The study assessed Cervarix® and Gardasil® administered either intramuscularly or intradermally, in different doses (full-dose or reduced to 20%) by different methods. The study concluded that ID administration of the vaccines not only increased the immune reaction, but also maintained safety while being tolerated well by the test subjects. The study suggests that further evaluation of ID HPV vaccination in areas with limited resources should be undertaken.9
For countries that face a shortage of vaccine, cold chain capability, or funding, the ID route provides an ideal alternative for administration that not only reduces cost, but also provides convenience for patients while maintaining optimum efficacy and safety. While the training required to administer can be significant, the aforementioned alternatives, including adapters and other delivery solutions, may help to increase the use of the ID injection as an alternative across the globe.
SUMMARY
Efficiency of vaccine use will be critical as the world population continues to grow and vaccine prices continue to rise. ID administration can help reduce dose cost while potentially improving immunogenicity in traditional and hard-to-treat populations. Advances in delivery systems have made consistency of administration into the intradermal layer possible without advanced training requirements and with minimal disruption to the pharmaceutical manufacturer. As more vaccine options reach the market, and costs continue to rise, ID administration may provide an excellent alternative to traditional vaccination without reducing the immune response.
REFERENCES
1. History of Vaccine Safety. Centers for Disease Control. http://www.cdc.gov/vaccinesafety/vaccine_ monitoring/history.html. Accessed 8/8/13.
2. Kim YC, et al. Delivery Systems for Intradermal Vaccination. Current Topics I Microbiology and Immunology, DOI: 10.1007/82_2011_123, Springer-Verlag Berlin Heidelberg, 2011.
3. Autologous Dendritic Cell Vaccine Loaded With Allogeneic Tumor Lysate Expression of Cancer Testis Antigens in Patients With Soft Tissue Sarcoma (ADCVCTAST). US National Institutes of Health Study. http://clinicaltrials.gov/ct2/show/NCT01883518?term=dendritic+cell+vaccine&rank=1. Accessed 8/8/13.
4. Aw D, et al. Immunosenescence: emerging challenges for an ageing population. Immunol. 2007;120(4): 435-446. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2265901/. Accessed on 8/14/13.
5. Flanagan KL, et al. The challenge of assessing infant vaccine responses in resource-poor settings. Expert Rev Vaccines. 2010;9(6):665-674. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2937226/. Accessed on 8/14/13.
6. www.clinicaltrials.gov. Accessed 8/14/13.
7. Verma R, et al. Intradermal administration of rabies vaccines in developing countries: at an affordable cost. Department of Community Medicine, Pt. BD. Sharma PGIMS, Rhotak, Haryana, India. http://www.ncbi.nlm.nih.gov/pubmed/21734465. Accessed 8/2/13.
8. WHO recommends the intradermal route for post-exposure prophylaxis in all places where rabies vaccines are in short supply. http://www.who.int/ith/vaccines/rabies/en/index.html. Accessed 8/2/13.
9. Ambrozaitis A, et al. Rabies post-exposure prophylaxis vaccination with purified chick embryo cell vaccine (PCECV) and purified Vero cell rabies vaccine (PVRV) in a four-site intradermal schedule (4-0-2-0-1-1): an immunogenic, cost effective and practical regimen. Infection Disease and Microbiology Department, Vilnius University, Vilnius, Lithuania. http://www.ncbi.nlm.nih.gov/pubmed/16545510. Accessed 8/2/13.
10. Nelson EA, et al. A pilot randomized study to assess immunogenicity, reactogenicity, safety, and tolerability of two human papillomavirus vaccines administered intramuscularly and intradermally to females aged 18-26 years. Department of Paediatrics, The Chinese University of Hong Kong, Hong Kong Special Administrative Region. http://www.ncbi.nlm.nih.gov/pubmed/23770335. Accessed 8/7/2013.

Zach Marks joined West in 2002 as Business Development Manager for the Clip’n’Ject reconstitution system. From 2003 to 2007, Mr. Marks held the additional position of Senior Account Manager covering strategic accounts, such as Amgen and Baxter BioScience. In 2007, with the acquisition of Medimop Medical Products, he was chosen for the position of Marketing and Business Development Manager for the group. During his tenure, his leadership and market knowledge helped expand the business into new accounts, products, therapeutic areas, and geographies. In 2010, Mr. Marks was appointed Director, Strategic Marketing and Innovation within the Delivery Systems Business Unit, where his combined experiences have been instrumental in establishing the marketing structure, function, and resources that have resulted in consistent growth of revenue and profitability. In 2012, in recognition of his contribution to the organization, his role was expanded to include business development and support of delivery system activities in Asia. Mr. Marks has prior experience including sales, marketing, and management roles with Johnson and Johnson and Waters Corporation. He is a registered Pharmacist with a BS in Pharmacy and an MS in Pharmaceutical Science from Rutgers University.
Total Page Views: 5527