Issue:January/February 2018
INTRABODY DELIVERY - The Feldan Shuttle Technology: A Peptide-Based Method to Deliver Antibodies
INTRODUCTION
Throughout the past decade, the use of monoclonal antibodies and antibody-related products invaded drug market with billion-dollar sale blockbusters (eg, Humira, Alirocumab, Evolocumab), which reflects their great therapeutic potential for human therapies.1-3 The number of biopharmaceutical antibodies in clinical drug development in 2015 increased 36% compared to 2014, with 53 novel products approved in Europe and the US for Phase 3 studies. Approximately 210 novel therapeutic antibodies are evaluated in the two earlier phases of clinical development to cure illnesses like asthma, multiple myeloma, Alzheimer’s disease, and psoriasis.4 While current antibody-based therapies aim at extracellular and transmembrane proteins, reaching the interior of cells with antibodies is still an interesting market to be explored because 60% of drug targets are believed to be intracellular. Indeed, there are many examples of intracellular proteins from nucleus, cytoplasm, and organelles (mitochondria, lysosomes, endoplasmic reticulum), which are suspected to be involved in disorders like oncogenic, autoimmune, and degenerative diseases.5 The therapeutic potential of antibodies with intracellular targets (“intrabodies”) was demonstrated with the insertion of coding DNA in mammalian cells that forced the expression of antibodies followed by the successful inhibition of cellular functions.6 However, the intracellular DNA expression of functional antibodies by mammalian cells is laborious and hard to translate to clinical applications. The direct delivery of intrabodies as proteins could become therapeutically interesting to specifically target signaling cascade components and to mediate instantaneous biological effects.5 However, the lack of safe and robust protein delivery protocols slows down the transfer of intrabodies toward widespread clinical use.5,7 In this review, we present the Feldan Shuttle technology, a peptide-based delivery method, that could provide the efficient and safe intrabody delivery in mammalian cells.
ADVANTAGES OF INTRABODIES OVER OTHERS PROTEIN CARGOES
The use of intrabodies as therapeutic molecules brings solutions to overcome challenges currently faced by the pharmaceutical industry with small molecules and antibodies targeting extracellular substrates. An antibody has the advantage, over classical small molecule drugs, to be highly specific for a single protein. They can be designed to discriminate between splice protein variants and even post-translational subpopulation (eg, a phosphorylated protein).6 This high level of specificity is accessible by using antibody fragments like antigen-binding fragments (Fabs) and single-chain variable fragments (scFvs). With the exponential growth of the therapeutic monoclonal antibody market, the technologies to design and produce antibodies enable antibody manufacturing scale up and are clinically approved. Clinical trials show that antibodies, which can be recombinantly produced and humanized, are generally well-tolerated and have high substrate specificity that significantly reduce the risk of deleterious side-effects compared to many others types of therapeutic products.3 Obviously, intrabodies opens new therapeutic avenues by their capability to bind intracellular proteins, which cannot be modulated by conventional antibodies or chemical drugs. The immediate but transient blocking effect of the targeted protein by intrabodies also provides an advantage over more indirect and less-specific RNA interference technologies or permanent modification provided by CRISPR nucleases.6
THE INTRABODIES DELIVERY CHALLENGE
Usually, proteins, including antibodies, do not have the innate capacity to penetrate cells by themselves in sufficient therapeutic concentration. Unfortunately, current viral and non-viral delivery methods (inactive virus, electroporation, chemical agents) have mainly been developed to introduce foreign genetic material but are poorly efficient to directly deliver proteins in cells, leaving few options for the delivery of purified antibody.8 Since antibodies engineering is a laborious process, especially to produce a specific and humanized monoclonal antibody, making intrabodies the new generation of therapeutics requires pressing and robust delivery methods well tolerated by mammalian cells and clinically acceptable.9
BRIEF REVIEW OF INTRABODIES DELIVERY INVESTIGATIONS
The feasibility of targeting intracellular proteins using intrabodies has been proven using either intracellular overexpression of antibody fragments through a gene-based approach or microinjection.10 In parallel, a few academic teams reported the use of electroporation to deliver antibodies.11 Even if electroporation could potentially be a relevant delivery method, this process is not efficiently applicable to in vivo therapeutic applications and notoriously leads to high mortality in many cell types. Few corporate and academic groups also reported that some cationic lipid agents could be used to translocate antibodies, but the toxicity associated with their use prohibits their transfer in clinical trials for both ex vivo and in vivo studies.12,13 Other protein-delivery techniques have been recently developed and could potentially be applied to intrabodies. For example, the use of a vector-free microfluidic platform to mediate physical force that “squeezes” cell membrane and leads to cell poration and passive protein uptake.14 In parallel, some laboratories modified the structure of antibodies to design engineered therapeutic antibodies in order to promote self-delivery, but the method is complex, requires important know-how, limits high throughput screening, and could influence the activity of the modified intrabodies.2 The following describes the Feldan Shuttle peptide-based technology as a robust method to enable quick and easy delivery of intrabodies in cells for both functional screening processes and therapeutic strategies.
THE FELDAN SHUTTLE TECHNOLOGY
Cell-Penetrating Peptides as Intrabody Carriers
Using short protein domains as intracellular carriers is a strategy discovered in 1965 that was rationally considered 30 years ago after the identification of the first cell-penetrating peptide (CPP), isolated from the HIV-transactivator TAT.15,16 Actually, several CPPs have been identified and fused to proteins to mediate their intracellular delivery. However, CPPs mediate intracellular uptake through endocytic processes that lead to the sequestration and the enzymatic degradation of proteins into vesicular compartments, named endosomes, that substantially reduces the likelihood to get functional effect.17
The Endosomal Escape Strategy
Indeed, the endosomal escape of delivered proteins is a challenging and limiting step that requires endosome destabilizing strategies.18 To overcome this hurdle, peptides with endosomal leakage properties could promote the cytosolic release of biomolecules from endosomes.19,20 These peptides, named endosomal leakage domains (ELDs), are unable to deliver proteins in cells on their own. Indeed, using both CPPs and ELDs could be an approach resulting in a peptide with both protein uptake and endosomal escape properties (Figure 1). Recently, the fusion of CPPs with ELDs was validated as a promising peptide-based delivery strategy with easily adaptable protocols for macromolecule delivery that provided exciting results into mammalian cells.21-23 These investigations encouraged us to deepen our understanding of the biochemical and structural properties required for the design of peptides mediating the efficient and safe delivery of proteins into mammalian cells.
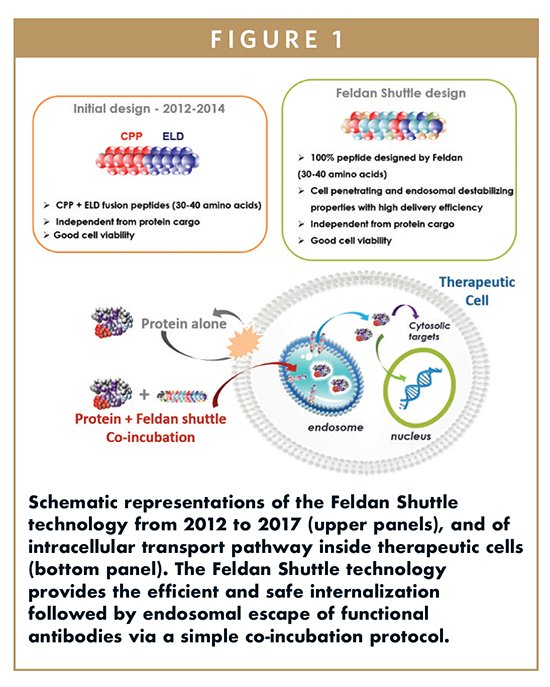
Toward a New Generation of Intrabody Carriers
The research division of Feldan aims at developing and patenting a new generation of peptides named Feldan Shuttles that provides the efficient, safe, and fast intracellular delivery of proteins, like antibodies, in several mammalian cells, including human stem cells, lymphocytes, myeloma, and primary cells. Initially based on the fusion of natural CPPs and ELDs sequences, Feldan peptides are now rationally designed and optimized for the delivery of intrabodies in living cells (Figures 1 & 2). Exempt from chemical modifications, the Feldan Shuttle is degraded after transient active use, a characteristic that considerably decreases regulatory burdens for human applications. Feldan Shuttle technology opens new avenues allowing the modification of hard-to-transfect cells with high therapeutic potential. These easily soluble designed peptides can deliver diverse antibodies in the cytoplasm of adherent and suspension cells using a simple co-incubation protocol. The Feldan Shuttles offer a high level of adjustment and accuracy to reach functional effects in cells. Indeed, this technology is in continuous improvement using both sequence analysis and computational learning approaches. Thereby, the Feldan Shuttle is a promising and effective peptide-based alternative to safely bring antibodies and other proteins in the cytoplasm of cells. Our main objective is to use the Feldan Shuttle platform to develop a high throughput technology that will bring a new strategy to target intracellular epitopes and to modulate signalling pathways, enabling the use of intrabodies as a drug to prevent, treat, or cure diseases.

INTRABODIES DELIVERY WITH THE FELDAN SHUTTLE TECHNOLOGY
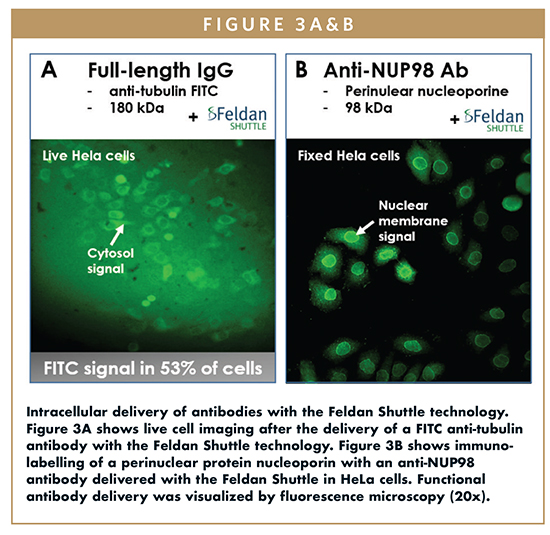
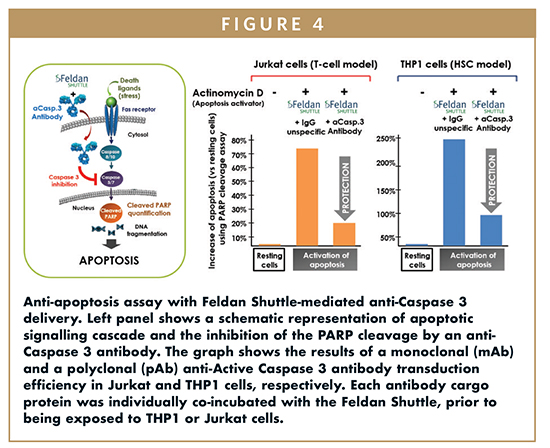
Initial results using the Feldan Shuttle demonstrates that intrabodies can be internalized while keeping their ability to bind a specific target to mediate cellular activity. The experiments presented in this review show that the Feldan Shuttle efficiently delivered the following functional antibodies: an anti-tubulin antibody (Figure 3A), an anti-NUP98 antibody that label the perinuclear protein nucleoporin (Figure 3B), and most importantly, two anti-Active Caspase 3 monoclonal and polyclonal antibodies that bind and inactivate the pro-apoptotic Caspase 3 protein in human monocyte THP1 and human immortalized CD4 T lymphocyte Jurkat (Figure 4).
Results in THP1 and in Jurkat cells showed that the Feldan Shuttle technology efficiently delivered the functional anti-Active Caspase 3 antibodies. Anti-TNF antibody was used as a non-specific IgG negative control, and cleavage of PARP protein was used as apoptosis measurement. In presence of the cytotoxic inducer actinomycin D, the Feldan Shuttle-mediated delivery of each anti-Active Caspase 3 monoclonal (mAb) and polyclonal (pAb) antibodies in THP1 and Jurkat cells, respectively, resulted in cell protective effect via the reduction of the basal level of apoptosis compared to the non-specific IgG antibody control condition. The simple and adaptable co-incubation protocol used here present many advantages to preserve cells from toxicity. Indeed, the Feldan Shuttle and each antibody were co-incubated with adherent or suspension cells only for 1 to 10 minutes, illustrating the fast delivery action mode of the technology. These results confirmed that the Feldan Shuttle technology efficiently and safely delivers functional intrabodies in mammalian cells.
FELDAN SHUTTLE TECHNOLOGY: APPLICATIONS & PARTNERSHIPS
Because the vast majority of therapeutic antibodies still aim at extracellular targets, the implementation of the Feldan Shuttle technology to deliver intrabodies in mammalian cells provides huge interests in the antibody market. The development of a Feldan Shuttle platform for the screening of functional intrabodies should result in the identification of intracellular protein targets with therapeutic interest. In the development process, multiple antibodies need to be generated against one target and subsequently screened for their specificity. Furthermore, this peptide-based strategy could be an alternative to optimize the use of small drug delivery molecules developed by industry/biopharmaceutical research that fail to localize therapeutically relevant protein-protein interactions inside the cell. The access to high-quality intrabodies will also allow Feldan Therapeutics to transiently modulate the cell machinery to get functional changes. Moreover, the Feldan Shuttle technology could deliver engineered intrabodies to transiently modulate signaling pathways at different cell cycle stages during cell proliferation and differentiation. This may be achieved with the delivery of specific intrabodies that could trap protein targets into specific intracellular compartments.5 For example, the intrabody-mediated transport of proteins into the nucleus could induce the specific and direct modulation of genome expression.6 The easy manipulation of this soluble and degradable peptide product could also be used for treatments requiring narrow time windows to protect brittle cells from toxic long-term exposure, and treatments requiring extended intrabody delivery to mediate more sustained biological effects.
The use of the Feldan Shuttle Technology for intrabody delivery can be directly used for ex vivo manipulation of mammalian cells to support the development of the screening platform. Its low cost of manufacturing, ease of use, and innocuity also provide this tractable method toward an industrial platform for the development of topical therapeutic products and local injections of intrabodies to treat problematics like skin inflammation in psoriasis, pain in osteoarthritis, and neovascular age-related macular degeneration.4 Because the in vivo delivery of intrabodies remains the ultimate goal for drug development, Feldan Therapeutics performed preliminary in vivo investigations with local injections of diverse recombinant proteins with the Feldan Shuttle in the brain and the muscle of rodents. Injected proteins diffused in tissues and provided functional activity, indicating the potential of this technology for in vivo applications. However, given the more complex environment, the systemic injection of the Feldan Shuttle product is still limited and needs improvements with peptide protection strategies like the use of peptide-coating agents and non-permanent peptide-cargo linking strategies. In this regard, Feldan Therapeutics continuously develops partnerships to seize exciting opportunities and expertise to combine the Feldan Shuttle technology with other approaches and to raise it toward intrabody-based clinical applications.
REFERENCES
1. Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. May 2009;157(2):220-233.
2. Chiu ML, Gilliland GL. Engineering antibody therapeutics. Curr Opin Struct Biol. Jun 2016;38:163-173.
3. Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7(1):9-14.
4. Reichert JM. Antibodies to watch in 2016. MAbs. 2016;8(2):197-204.
5. Miersch S, Sidhu SS. Intracellular targeting with engineered proteins. F1000Res. 2016;5.
6. Marschall AL, Dubel S. Antibodies inside of a cell can change its outside: Can intrabodies provide a new therapeutic paradigm? Comput Struct Biotechnol J. 2016;14:304-308.
7. Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J Pharm Sci. Jun 2010;99(6):2557-2575.
8. Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF. In vitro and ex vivo strategies for intracellular delivery. Nature. Oct 13 2016;538(7624):183-192.
9. Ford KG, Souberbielle BE, Darling D, Farzaneh F. Protein transduction: an alternative to genetic intervention? Gene Ther. Jan 2001;8(1):1-4.
10. Keppeke GD, Andrade LE, Grieshaber SS, Chan EK. Microinjection of specific anti-IMPDH2 antibodies induces disassembly of cytoplasmic rods/rings that are primarily stationary and stable structures. Cell Biosci. 2015;5(1):1.
11. Marschall AL, Zhang C, Frenzel A, et al. Delivery of antibodies to the cytosol: debunking the myths. MAbs. Jul-Aug 2014;6(4):943-956.
12. Courtete J, Sibler AP, Zeder-Lutz G, et al. Suppression of cervical carcinoma cell growth by intracytoplasmic codelivery of anti-oncoprotein E6 antibody and small interfering RNA. Mol Cancer Ther. Jun 2007;6(6):1728-1735.
13. Matsushita S, Takahama S, Shibata J, et al. Ex vivo neutralization of HIV-1 quasi-species by a broadly reactive humanized monoclonal antibody KD-247. Hum Antibodies. 2005;14(3-4):81-88.
14. Sharei A, Zoldan J, Adamo A, et al. A vector-free microfluidic platform for intracellular delivery. Proc Natl Acad Sci USA. Feb 05 2013;110(6):2082-2087.
15. Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. Dec 23 1988;55(6):1189-1193.
16. Ryser HJ, Hancock R. Histones and basic polyamino acids stimulate the uptake of albumin by tumor cells in culture. Science. Oct 22 1965;150(3695):501-503.
17. Guidotti G, Brambilla L, Rossi D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol Sci. Apr 2017;38(4):406-424.
18. Akishiba M, Takeuchi T, Kawaguchi Y, et al. Cytosolic antibody delivery by lipid-sensitive endosomolytic peptide. Nat Chem. 2017;advance online publication.
19. Reinhardt A, Neundorf I. Design and Application of Antimicrobial Peptide Conjugates. Int J Mol Sci. May 11 2016;17(5).
20. Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. May 10 2011;151(3):220-228.
21. Luan L, Meng Q, Xu L, Meng Z, Yan H, Liu K. Peptide amphiphiles with multifunctional fragments promoting cellular uptake and endosomal escape as efficient gene vectors. Journal of Materials Chemistry B. 2015;3(6):1068-1078.
22. Salomone F, Cardarelli F, Di Luca M, et al. A novel chimeric cell-penetrating peptide with membrane-disruptive properties for efficient endosomal escape. J Control Release. Nov 10 2012;163(3):293-303.
23. Lee YJ, Erazo-Oliveras A, Pellois JP. Delivery of macromolecules into live cells by simple co-incubation with a peptide. Chembiochem. Feb 15 2010;11(3):325-330.

Dr. Thomas Del’Guidice is a Research Specialist at Feldan Therapeutics. His work focuses on the development of the Feldan Shuttle, a peptide-based technology for the intracellular delivery of native and engineered proteins. He earned his PhD in Molecular and Cognitive Neurobiology from the Laval University, Québec-Canada. He achieved an industrial post-doctoral fellowship at Feldan Therapeutics in the design and the screening of peptide sequences for the intracellular delivery of recombinant proteins and drug reagents in mammalian cells.

Dr. Nancy Messier is a Development Director at Feldan Therapeutics. She earned her PhD in Microbiology from the Laval University, Québec-Canada for her research on the implication of the class 1 integrase structure-function in bacterial antibiotic resistance. She has more than 10 years of expertise in the analysis and the production of recombinant proteins and leads the development of the intrabody delivery project at Feldan Therapeutics.

Dr. David Guay is Research Director at Feldan Therapeutics. He earned his PhD in Molecular and Cell Biology from Laval University, Québec-Canada for his research on the implication of a transcription factor in DNA repair and chemotherapy-resistance mechanisms. He held a post-doctoral fellowship from McGill University in chemical engineering, where he developed a DNA delivery technique based on a non-thermal plasma technology. His research at Feldan Therapeutics focuses on the Feldan Shuttle, a peptide-based technology designed to deliver native proteins, namely antibodies and CRISPR nucleases, in therapeutic cells.
Total Page Views: 6791