Issue:September 2015
HOT MELT EXTRUSION - OptiMelt™ Hot Melt Extrusion Technology to Improve Bioavailability of Poorly Soluble Drugs
INTRODUCTION
One of the biggest challenges faced today by the pharmaceutical industry lies in enabling the delivery of difficult-to-solubilize molecules. Solubility is an essential physico-chemical characteristic of active pharmaceutical ingredients (APIs), as it directly relates to bioavailability of the molecule internally in the body. Poorly soluble APIs dissolve/disperse sparingly in the gut and have generally low bioavailability. Approximately 40% of currently marketed drugs are classified as poorly soluble (BCS Class II/IV), and more than 70% of drugs in development are also poorly soluble, representing an increasing industry challenge, especially so given that it can be quite resource intensive to develop formulations for poorly soluble drugs. The problem is most severe when the molecule has both low solubility and a high dose.
To accelerate the development of molecules through the clinical programs, technologies for mitigating the effects of the poor solubility and in turn poor bioavailability are becoming essential for the success of new drug molecules.
Hot melt extrusion (HME) is a technology that is gaining interest in the pharmaceutical industry as a novel technique to generate physically stable and processable amorphous forms of APIs. Relative to crystalline APIs, amorphous solid dispersions improve bioavailability in more than 80% of cases, thus enhancing R&D productivity and enabling effective drug development. The mechanism of HME is to disperse APIs in the polymer matrix at the molecular level to form solid dispersions or solid solutions. The main driver of increased use of HME technology in the pharmaceutical industry is its capability of continuous manufacturing with solvent-less processing and the versatility of the downstream processing of extrudates into final dosage forms (tablets, capsules, controlled release forms, stick packs etc).
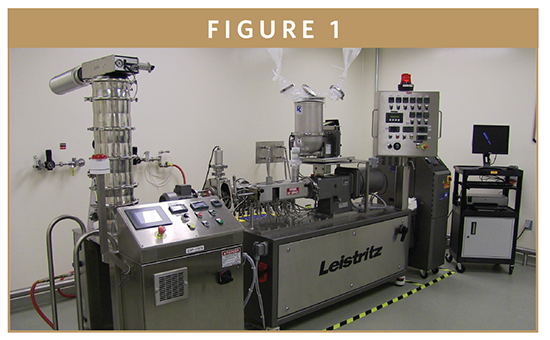
QUALITY-BY-DESIGN
HME enables product development driven by Quality-by- Design (QbD). Thus, parameters including degree of dispersion, level of impurities, extent of dissolution, stability, and morphology are optimized through the application of an extrusion process, in which characteristics such as residence, time, and shear stress, are controlled via a number of input variables. These include feed rate, temperature, screw design, screw speed, and the physical properties of the materials.
Selection criteria for the polymeric carrier to be used include its interaction with the drug; the potential for supersaturation; and the solid-state solubility of the drug in the carrier. Important polymer characteristics include the design of the manufacturing process, the solubility of the polymer in water and organic solvents, its molecular weight, and its glass transition temperature (Tg). Ideally, the polymer matrix acts as a solid solvent, and the drug is molecularly dissolved. To achieve this, the polymer should have good thermoplastic behavior (deformability is essential); thermal stability (Tg from 50°C to 180°C); low hygroscopicity to prevent crystallization; no toxicity, so that it may be used in large amounts; and either high or no solubility to ensure thermodynamic stability.
To develop suitable formulations, it is desirable to keep the extrusion formulation and process as simple as possible through the choice of a suitable polymer; the use of a plasticizer for improved solubility only if necessary; and the use of a solubilizer for improved drug content and prevention of crystallization in gastric and intestinal fluid.
The quality of the extrudate is determined through the application of a number of analytical techniques, including optical microscopy to determine sample surface morphology and the presence of crystalline particles; scanning electron microscopy (SEM) higher-resolution imaging; and atomic force microscopy (AFM) to provide three-dimensional images. Other analytical techniques commonly used to determine the quality of the API/polymer extrudate include differential scanning calorimetry (DSC), X-ray diffraction crystallography (XRD); solid-state nuclear magnetic resonance (ss-NMR); and infra-red and Raman spectroscopy.
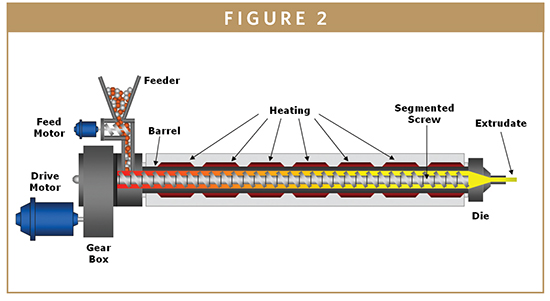
INTEGRATED APPROACH TO ENHANCING BIOAVAILABILITY
Catalent and BASF have formed an open alliance in which scientists from the two companies are working together to deliver optimal solubility solutions, thus saving customers time, money, and resources. API and preformulation studies combine Catalent’s Optiform® salt screening API optimization technology with solubilizers and refinements proposed by BASF. Drug formulations are designed through combining Catalent’s expertise in polymer screening for HME processing with BASF’s Solu-HTS high throughput solubilizer screening and polymer selection expertise; HME lab-scale processing is accomplished through combining Catalent’s lab-scale HME (OptiMeltTM) and RP Scherer Softgel/OptiShellTM screening technologies with BASF’s experience in polymer and formulation optimization. Scale-up and downstream processing, including HME scale-up, tableting, coating, and encapsulation, is performed by Catalent using binders, disintegrants, and coatings supplied by BASF and by applying its own expertise in formulation optimization.
ENABLING HOLISTIC BIOAVAILABILITY ENHANCEMENT
An integrated end-to-end HME process provides the capability to formulate, develop, and commercialize differentiated final dosage forms. It enables holistic bioavailability enhancement by broadly addressing multiple bioavailability factors, including optimization of product efficacy, safety, and release properties. Beginning with initial API and preformulation studies, the required formulations can be developed through the application of a range of formulation screening technologies and analyses.
To maximize the efficiency of HME extrusion processes, predevelopment laboratory studies are necessary. These include API thermostability, including DSC of binary mixtures to identify the Tg of the API; hot-stage microscopy to observe phase melting/dissolution at different temperatures; miscibility studies using predictive or small-scale experimental techniques (film casting and hot plate); and film casting of binary mixtures from a common solvent and visual examination for crystal formation process simulation. The properties of an API determined in such studies, including Tg if available or otherwise estimated (Tg/Tm [Kelvin] ratio ~0.7 based on fragility theory), API chemical stability at increased temperature, and API availability for hydrogen-bonding, direct formulation strategy and the subsequent establishment of a development plan.
HME high-throughput processing gives rapid proof of concepts and is energy-efficient; continuous processing gives rapid scalability to commercial supply and reduces waste; and solvent-free processing eliminates solvent-API interactions, residual solvent risk and solvent handling.
Final dosage form development comprises the HME process followed by pelletizing or milling. The extrudate of API solid dispersion is prepared in a polymer/excipient matrix, the process being typically co-located with downstream processing and solid dose form manufacture. Process development and optimization is performed on various sizes of extruder, and includes Design of Experiments (DoE) trials, production of prototype batches, formulation transfer and scale-up, before progressing to full-scale GMP production. Auxiliary equipment within a manufacturing facility would usually include a gravimetric feeder, cooling conveyor, milling facilities, a multi-cut strand pellitizer, and a mini-calender. However, the exact nature and specifications of these would vary depending on customer specifications for final dose forms.
HME allows great flexibility and variation in the final dose form. Tablets, either uncoated or coated, with the option of multi-layered formulations, are possible, as is the option of formulating products with controlled-release properties. Powders, beads, or granules can be manufactured to be formulated into capsules or packaged as “stick pack” sachets with free-flowing contents.
IMPROVED THERAPEUTIC PROFILES
HME technology improves therapeutic profiles of new drugs in a number of ways and enables treatment optimization, the wide range of solubilities and dispersion concentrations possible giving the flexibility to achieve desired efficacy and dosing. The very high drug loading possible (up to 90%) gives a reduced daily pill burden and enhances patient compliance, while patient abuse-deterrence formulations also enhance patient safety and compliance. In addition, the greater consistency for APIs with high food effect absorption gives enhanced efficacy and reduces patient variability, and the taste-masking capability achievable with an HME formulation also contributes to product differentiation while enhancing patient compliance.
CASE STUDY 1: INCREASED BIOAVAILABILITY OF A CHRONIC INFLAMMATORY DISEASE DRUG
The following case study demonstrates how OptiMelt technology increases bioavailability and shortens time to market. The challenge was that a poorly bioavailable product for treating chronic inflammatory disease required effective formulation for Phase II development. Formulation, process development, and downstream tableting were all required. Using OptiMelt technology, Catalent developed and optimized an effective tablet formulation through the application of:
-HME polymer screening and selection
-Drug/polymer formulation development
-Determination of HME processing parameters
-Scale-up from feasibility to development using a 10-mm extruder (50 g) to 18-mm extruder (4 kg)
-Downstream tableting, including extrudate/tablet formulation and tableting operating parameters selection
OptiMelt enabled effective Phase II development of the customer’s candidate.
CASE STUDY 2: ENHANCING BRAND PERFORMANCE OF AN OTC ASPIRIN PRODUCT
In another example, Catalent applied OptiMelt technology to enhance the product and brand performance of an OTC aspirin product. The challenge was that a large company wanted to develop and launch an effervescent aspirin OTC product as a line extension to a very well-recognized brand. Catalent partnered with the company for product formulation, scale-up, and commercial manufacturing to provide:
-An innovative effervescent aspirin formulation
-HME process development
-Downstream processing in which the product was milled, mixed, and filled into a unique and distinct single-dose packaging solution stick packs
-Scale-up and commercial manufacturing
The result was a successful product launch with a unique formulation, complementing the leading analgesic OTC brand.
SUMMARY
Bioavailability enhancement with formulation and dose form flexibility can be achieved through the application of HME technology to produce stable drug formulations and increased development success rates. The wide range of dose functionality achievable allows immediate- and controlled-release formulations to deliver more active drug, when and where it is needed in the body. The multiple possibilities of dose forms available with the technology, including tablets, capsules, and free-flowing granules, results in a preferred patient dose form, thus enhancing patient compliance and providing product differentiation.
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Sampada Upadhye is Technology Platform Leader for Bioavailability Enhancement and OptiMeltTM at Catalent Pharma Solutions. She earned her PhD at The University of Mississippi under the supervision of Dr. Michael A. Repka, where her graduate research focused on hot melt extrusion of highly degradable new chemical entities. Before joining Catalent, she worked for Colorcon, Inc. and Pfizer, where she gained extensive experience in the area of solubilization of preclinical NCEs and formulation design of amorphous solid dispersions using hot melt extrusion and spray drying technologies. Her ongoing research interests include formulation design and process development of extruded formulations with twin screw technology. Dr. Upadhye is the author of a number of publications and has contributed chapters to the following books: Hot Melt Extrusion: Materials, Technology and Drug Product Design (Edited by MA Repka, et al) and Contemporary Research Topics in Pharmaceutical Excipients (Edited by Ajit Narang, et al).
Total Page Views: 6381

















