Issue:May 2017
CLINICAL RESEARCH - Behind the Wave: Clinical Research in a Digital Transformation Era
INTRODUCTION
Over the past few years, many well-established industries have seen rapid and disruptive innovations. Some say this transformation began with the internet, which allowed faster information sharing all over the world. Others argue that the Apple iPhone fueled this change by putting mobile connectivity into the pockets of consumers. These technologies allowed service innovations that earlier would have sounded far-fetched. Who would have guessed just 10 years ago that AirBnB would enable consumers to compete with huge hotel chains? Or that Uber drivers can now compete with taxis? Meanwhile, many traditional industries like printing, paper photography, and entertainment have had to reinvent themselves in order to survive.
While this digital transformation wave is changing the world, what has changed in the clinical trial model? Not much. Sure, some forward-thinking companies use online recruitment as a mechanism to attract more patients into trials. However, the “innovation” often ends with the interested candidate clicking on a web link where they are told to call to learn more. For the consumer accustomed to working online, this can be a disappointing user experience.
Perhaps even more discouraging is the fact that study sites often deal with the same problems they have long had. Granted, we’re no longer shipping laptops to sites loaded with data capture technology, but shipping handheld devices, smartphones, and tablets to sites remains common practice. Additionally, sites still rely on several complicated systems with overlapping functionality that all have different usernames and passwords and that require the same information to be keyed in over and over again. These problems also extend to the sponsors who do not have access to real-time information, spend hundreds of hours reconciling data streams from different systems, and have little information available to proactively manage their clinical research to avoid delays and operational issues in the study.
The remote research model and purpose-built technology offer the tools and the right process to help modernize clinical research and bring it closer to the high standard set by today’s consumer technologies. The following will address key aspects of the model and technology from the perspective of the different stakeholders in a clinical trial: the patients, the sites, and the sponsors.
PATIENT-CENTRIC VIEW
Patient-centricity is certainly one of the hottest buzzwords in the pharmaceutical industry today. Many companies are taking concrete action, and these efforts are further fueled by regulators. For example, the US Food and Drug Administration created a Patient-Focused Drug Development Program and has been vocal about patient-centricity in drug development. Many companies use patient focus groups to gain insight. Several companies have even developed patient-centricity toolkits with software and service offerings that study teams can utilize in their own programs.
However, before anyone can claim they are delivering patient-centric clinical development programs, they must truly understand the views of the patients.
Clinical research is typically conducted by highly educated people who have an in-depth understanding of the medical condition in question and the mechanism of the drug being researched. They understand the evidence that regulators expect in order for the new product to be approved. But do they understand the patient perspective? In reality, because of the way randomized clinical trials are conducted and because of patient privacy requirements, the sponsor’s study teams may rarely speak with patients.
So what options does the sponsor team have to hear the patient’s voice? While focus groups can be good and provide a bi-directional mechanism to discuss issues with patients to obtain qualitative input, they are limited. They often provide a small demographic sample size, are impractical for global trials, and by nature, only provide a snapshot view rather than a continuous feedback loop.
A patient-engagement technology solution can address these issues. As a trusted third-party solution, eClinicalHealth’s Clinpal platform can facilitate a purpose-built patient community globally that can be continuously engaged during study design, recruitment, and conduct, ensuring that patient feedback is part of the end-to-end process. Such a platform, combined with the reach of online and social media, can be put into place quickly in a global setting to gather insights from hundreds of individuals of different cultures. Also, because potential candidates are accustomed to regular online communications (nearly three-quarters of US internet users look online for health information) new technology makes it even more easy for patients to become engaged.1
The patient feedback loop can be implemented in a completely remote setting, but it does not need to end there. Nearly any study can use technologies such as Clinpal to make trials more convenient and efficient for patients. Furthermore, patient recruitment is part of the consenting process, which can be supported with technology as well by using online advertising and patient-engagement technology to reach out to potential candidates. This can aid online referral processing, supported by an interactive patient dashboard with status information, study documents, and secure messaging functions. Study candidates learn about the study through remote access to the patient information sheet or even through a completely electronic informed consent, where the candidate can electronically sign the document before the first study visit. Patients and sites both benefit by:
– Avoiding unnecessary and sometimes inconvenient in-person study visits
– Enabling the patient to dedicate more time learning about the study and discussing it with family members without feeling “rushed” during a site visit
– Making study visits more efficient for all because study details are already understood by the candidate before the first visit
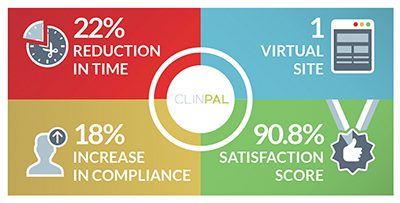
– Improving compliance and speeding study conduct: A remote trial conducted with Clinpal showed an 18% improvement in compliance and a 22% faster completion time2
Another excellent tool for improving patient engagement in clinical trials is to give patients access to their own data. In the remote VERKKO study, a Phase IV clinical trial for diabetes, patients had access to a logbook view of glucose readings they took using a smart, wireless glucose meter. The logbook indicated when readings were done at the right times and what the values were. Once they completed the required number of readings, they had access to a full report that showed their disease state at the various daily time points.3 When patients understood the study protocol and expectations and were provided with information about how they were doing, they could manage their own compliance, which was likely a key factor in the improved compliance and faster completion time.
Patient involvement and feedback should be a process, not a milestone. Patients can be engaged at strategic points during the trial by inquiring about their satisfaction and requesting suggestions. For example, at the end of the enrollment process, patients not enrolled could be asked the key reasons they did not take part. Patients who were enrolled can be asked how they feel about the upcoming study and also asked if they have any questions.
Using a trusted third-party system, this process can involve patients directly without burdening the study sites. This process was used in the VERKKO study: Patients shared information willingly and provided meaningful insights. In this case, patients suggested improvements to patient-facing instructions as well as some things that would have made participation more convenient. Several patients also shared feedback about products and how these relate to their lives.
THE SITES’ PERSPECTIVE
Many study sites suggest that clinical trials are becoming more time consuming and cumbersome, concerned about the time spent on administration rather than on core study activities, such as working with patients and assisting with clinical procedures required by the protocol. Study resourcing can also be challenging.
Remote trial methodologies address many of these issues. While enabling patients to conduct more of the study activity remotely does not necessarily mean less work, it does mean the work can be conducted more flexibly. For example, patients with access to convenient and remote messaging tools are more likely to interact with the site than if they must rely on study visits or restricted telephone hours. These patients also will expect prompt responses. However, the work of responding to patients can be distributed to several parties as it is no longer tied to physical location or time. First-level patient communications can be delegated to a call center, for example, which can triage requests and work with sites when the issue calls for the sites’ involvement. These duties can also include routine patient compliance monitoring and contacting those who need extra support.
In this way, remote trial technology can significantly reduce sites’ workload, allowing them to enroll more patients with the on-site resources they have. In the aforementioned remote VERKKO study, the study nurse reported that overall patient management required only one-third the effort necessary in a previous study with a similar protocol. Dr. Vehkavaara, the VERKKO study investigator, concluded, “This study was the most convenient clinical diabetes trial I have ever participated in.”
Remote clinical trial technology and processes are not a threat to traditional clinical trial sites and study coordinators. Instead, they enable sites to conduct studies more efficiently, allowing them to take part in more trials and enroll more patients with existing resources. Furthermore, study coordinators will likely find their role more meaningful as the administrative burden is decreased and they have better tools to communicate with and get direct feedback from patients.
OPPORTUNITIES FOR STUDY SPONSORS
Clinical trial sponsors are often driven by factors involving time, cost, and quality. Let’s evaluate the benefits of remote trial methods from the perspective of these three items.
Time
Time-to-market is critical in the pre-approval stage of a clinical program. Time spent on study conduct eats into patent protection time; beating the competition to the market is essential or the whole drug approval might be at risk. There is often very little that can be done to compress the timeline defined by the protocol; however, there is much that can be done during the study design, start-up and recruitment phases to incorporate patient insights and speed up the overall trial timeline. While there is often much focus on the first-patient-in date in a clinical trial, the more important date is the last-patient-in date, which often determines the trial’s end date. Remote methodologies can help get studies to this point faster by making start-up, recruitment, and communication more efficient.
In the post-approval phase when the product is already on the market, it is vital for companies to support the product launch with more data. Post-approval studies are often required, and there are market research and data demanded by payers for pricing and reimbursement. By deploying remote studies, companies can launch these time-critical post-approval studies quickly. Because they utilize flexible technology rather than manual site-based procedures, these remote post-approval studies can also be adjusted rapidly. Online advertising outreach, electronic informed consent, and remote data capture processes are key tools these remote technologies use to engage large populations quickly.
Cost
Remote trials are cost-efficient and very scalable, making them even more attractive for large observatory, real-world trials. Many of the key aspects that remote trials address are also some of the biggest ticket items. Patient recruitment and site start-up costs can be decreased by a remote model in any phase of drug development. However, the optimal fit for the remote model is really in the population level or registry trials that often involve tens of thousands of patients. These trials can benefit from a high degree of automation that minimize the touch points with sites; even very small process improvements can mean huge reductions in the overall study cost.
Single-platform technologies that can support the entire trial conduct can also drive costs down by minimizing data discrepancies and decreasing overall cost of ownership when compared to the typical disjointed IT infrastructure utilized in studies.
Quality
As noted earlier, remote trials are often more patient-centric because they deploy efficient methods and technologies to support patients. Participants who are better informed and more engaged are also more likely to be compliant with the study protocol and less likely to drop out – ultimately resulting in better data quality. Moreover, utilizing a patient feedback loop and real-time metrics across the study conduct enables a completely new way of running clinical trials, one that is based on real-time information in a single database rather than offline reports based on outdated information. Remote trial methods and technologies provide access to real-time recruitment analytics, patient satisfaction metrics, study throughput flow, and protocol compliance. Any bottlenecks or issues will be identified early on, enabling the sponsor to make data-driven decisions on a daily basis to ensure successful trial conduct.
HARNESSING THE POTENTIAL OF REMOTE TRIAL METHODOLOGY
The aforementioned technologies are readily available and proven today. Regulators are supportive of these initiatives and are actively working on programs and guidance for the industry. The sites are on board, and remote trial methods have been proven with patients. Robust technology exists to support such programs, and the benefits are clear. So why hasn’t the industry seen more remote trials?
A lot has to do with change management. Sponsor companies may not have sufficient internal expertise to operationalize such innovations, and there are only a few vendors and technology solutions that support the clinical trial process end to end. eClinicalHealth and its Clinpal platform have been built from the ground up to support this need and have the in-house expertise and partner network to fully implement remote trials. The VERKKO trial was an important milestone in validating the approach and gathering satisfaction and performance metrics across the conduct of the trial. It is also important to understand that not every trial is suitable for a remote methodology, but that nearly every trial has at least some aspects that can benefit from these methods.
Similarly, it is critical to understand technology alone won’t put this industry ahead of the digital wave; technology must be used only if it makes trial conduct easier. We cannot expect sites to manage whatever the latest technology platforms sponsors or contract research organizations wish to deploy. And, it is inconvenient and unwieldy for patients to lug around site-provided smartphones, tablets, or laptops and cumbersome for sites to manage. This is one reason the industry continues to seek bring-your-own-device (BYOD) solutions. For example, Clinpal enables patients to use their own devices; when the prescreening process reveals that a patient doesn’t own an appropriate device, then devices are procured as necessary.
It is important to analyze each protocol and think of practical ways to apply these new methods. Our goal must be to involve patients in real ways while reducing site burden. Only when we deploy an effective, purpose-built, and single-platform technology will we truly modernize clinical research. Only then will we be able to realize the benefits of faster patient recruitment, improved patient engagement, faster time-to-market, and lower costs.
REFERENCES
1. Pew Internet & American Life Project survey, September 2012. http://www.pewinternet.org/factsheets/health-fact-sheet/.
2. Clinpal press release, June 16, 2016.http://www.clinpal.com/clinpalblog/eclinicalhealth-announces-successful-results-entirely-remote-onlineclinical-trial-3/.
3. eClinicalHealth co-founders on challenge of making remote monitoring for clinical trials manageable, MedCity News, June 27, 2016. http://medcitynews.com/2016/06/patient-engagement-and-clinical-trials/?rf=1.

Kai Langel is the Director, Patient Solutions and Co-Founder of eClinicalHealth. Since 2000, he has been a pioneer in patient-facing systems for clinical trials. Through his involvement in technical, operational, and scientific roles, he has gained an in-depth understanding of all aspects of the patient journey in clinical trials from recruitment and engagement through data capture. He is actively involved in providing guidance to eClinicalHealth’s customers on how to best operationalize new and innovative methods for making it easier and more efficient for sites and patients to participate in clinical trials. Mr. Langel is a respected leader in the industry and frequently speaks at industry conferences. He has authored several articles targeting eClinical working practices and lessons learned.
Total Page Views: 5103

















