Issue:March 2017
CELL THERAPY - PLX-R18: Cell Therapy for Treatment of Acute Radiation Syndrome & Bone Marrow Failure Diseases
INTRODUCTION
PLacental eXpanded (PLX) cells are placenta-derived, ex vivo, expanded, mesenchymal-like, adherent, stromal cells that are designed to be administered to patients without the need for tissue or genetic matching. These cells, expanded on the world’s first GMP-approved 3D bioreactor cell growth platform, release soluble biomolecules that act in a paracrine or endocrine manner to facilitate healing of damaged tissue by stimulating the body’s own regenerative mechanisms.
PLX-R18 cells, Pluristem’s second PLX product, release a combination of therapeutic proteins in response to a damaged or poorly functioning hematopoietic system, the system that creates the blood cells that protect us from infection, uncontrolled bleeding and anemia. The product is currently in development to treat Acute Radiation Syndrome (ARS) and incomplete engraftment of transplanted hematopoietic cells.
ARS is a syndrome caused by exposure to a high dose of ionizing radiation over a short period of time; even low doses of radiation damage the radiosensitive hematopoietic system (causing H-ARS). To date, FDA-approved therapies for treatment of HARS include single proteins, such as G-CSF and GM-CSF.1 Cell therapies have the potential to rescue hematopoietic failure based on a complex mechanism of action, involving numerous secreted proteins released from cells that react to their in vivo environment. Pluristem has tested the ability of a 3D-expanded placenta-derived stromal cell product, PLX-R18, designated for the treatment of hematological disorders, to alleviate symptoms in the H-ARS mouse model. Studies support the conclusion that PLX-R18 is a strong candidate for the treatment of H-ARS as well as a plethora of bone marrow failures with similar symptomatology.
PLX-R18 ACTIVITY IN VITRO
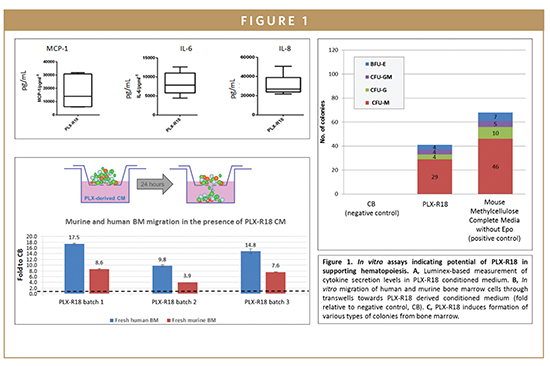
In vitro, PLX-R18 cells were shown to secrete hematopoietic proteins, proteins involved in maintenance, renewal, differentiation, and mobilization of hematopoietic cells, such as GCSF, MCP-1, IL-6, and IL-8. In addition, the secretions from PLX-R18 cells have been demonstrated to induce bone marrow migration through transwell inserts and to stimulate colony formation. Therefore, their ability to secrete regenerative proteins that positively impact the hematopoietic process supports their potential as therapeutics for indications involving failure of the hematopoietic process.
PLX-R18 RESCUE OF HEMATOPOIESIS IN VIVO
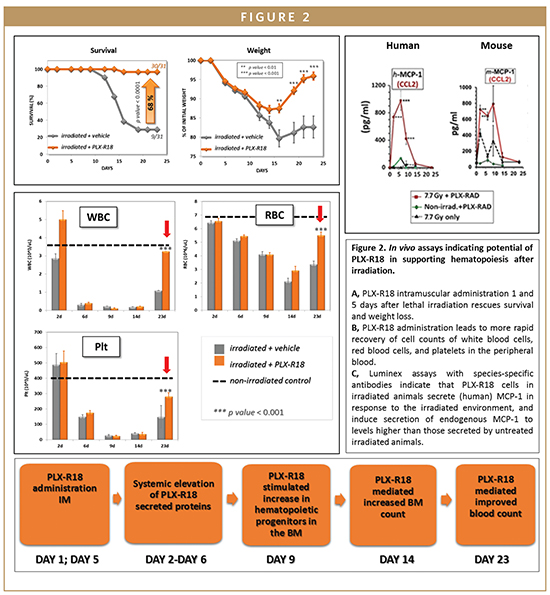
PLX-R18 efficacy in rescuing hematopoiesis was tested in a well-characterized model of lethal irradiation in collaboration with NIAID and with Hadassa Medical Center. Administration of PLXR18 intramuscularly to mice 1 and 5 days after total body irradiation (LD70/30) significantly increased survival and rescued-radio-induced weight loss relative to vehicle-injected controls. Similar results were seen when cells were injected on days 2 and 5 after irradiation, indicating that a window of time exists during which therapy can still be effective in case of lethal irradiation.2 In addition, cell treatment significantly increased the number of colony-forming hematopoietic progenitors in the bone marrow and raised peripheral blood cellularity to values near those of un-irradiated control values.
In vivo secretion studies indicate that PLX-R18 cells responded to radiation-induced hematopoietic failure by transiently secreting haematopoiesis-related proteins to enhance reconstitution of the hematopoietic system. The PLX-R18 cells secreted factors that induced the early secretion of endogenous (mouse-derived) hematopoietic factors (such as KC, IL-6, and G-CSF) in irradiated mice. These factors were secreted on days 2-14 after irradiation in treated mice (peaking on day 4-9), whereas in untreated animals they peaked much later (at around day 16) in those animals which survived to this time point. Secretion of exogenous (PLX-R18-derived) and endogenous (mouse derived) factors enables an earlier increase in the number of multi-lineage hematopoietic precursor cells (HPCs) in the bone marrow and enables the migration of bone marrow cells. Higher levels of cellularity in the bone marrow can be seen on days 4-9 following PLX-R18 treatment, allowing earlier hematopoietic rescue after irradiation. Proliferation, differentiation, and migration of HPCs ultimately leads to an elevation in the levels of multiple blood lineages in the peripheral blood (with significant differences visible on day 23 after treatment). The end result of this regenerated hematopoietic system is a higher survival rate in PLX-R18-treated irradiated mice.
PLX-R18 SAFETY
In vitro studies have indicated that PLXR18 lacks the ability to differentiate into osteocytes or adipocytes, supporting their proposed mechanism of action of endocrine protein secretion. PLX-R18 cells were proven to have a stable karyotype and to reach senescence, supporting their safety for clinical use. PLX-R18 cells do not express HLA class II molecules or co-stimulatory molecules, supporting their use as an off-the-shelf allogeneic product. Safety studies in vivo have indicated no treatment-induced toxicity or pathologies, and biodistribution studies indicate that the PLX-R18 cells remain localized to the site of injection within the muscle. Therefore, clinical use of PLX-R18 by intramuscular administration is not expected to have any associated safety concerns.
Taken together, placenta-derived stromal cells have the capacity to alleviate bone marrow failure symptoms arising from acute radiation by systemic secretion of proteins. PLX-R18 can be safely used as an off-the-shelf allogeneic treatment, enabling Pluristem to harness the power of cell therapy to provide next-generation treatment options for ARS. This new generation of therapies secretes multiple proteins with hematopoietic potential and naturally responds to the in vivo environment in real-time. The US National Institutes of Health’s NIAID is initiating dose evaluation studies of PLX-R18 in ARS as a basis for a potential pre-marketing trial in a large animal model.
To view this issue and all back issues online, please visit www.drug-dev.com.
REFERENCES
1. Singh VK, Newman VL, Seed TM. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine. 2015;71:22-37.
2. Gaberman E, Pinzur L, Levdansky L, Tsirlin M, Netzer N, Aberman Z, Gorodetsky R. Mitigation of Lethal Radiation Syndrome in Mice by Intramuscular Injection of 3D Cultured Adherent Human Placental Stromal Cells. 2013;PloS one 8 e66549.

Dr. Racheli Ofir joined Pluristem in 2007, serving as Vice President of Research and Intellectual Property. She is responsible for leading projects involving the characterization of PLX cells, Pluristem’s leading placenta-derived cell product candidate, including evaluating the biological activity of the cells in in vitro and animal studies. She is also responsible for the studies that determine the safety profile and pharmacokinetics of PLX cells and is in charge of all preclinical aspects of the PLX regulatory process and for the communications with the relevant regulatory authorities. She has applied and prosecuted over 10 patent families filed worldwide, is co-inventor on five patent applications, and is coauthor of numerous peer-reviewed articles. Dr. Ofir earned her PhD from the Technion, Israel Institute of Technology, where she investigated Telomeric position effects on gene expression and DNA replication in Human cells. She completed her postdoctoral fellowship at the Technion in Molecular Embryology, investigating genes involved in early development of vertebrates’ nervous system.

Dr. Noa Sher is the Product Profile Research Manager at Pluristem Therapeutics, responsible for all in vitro and in vivo studies involving mechanism of action, safety, and efficacy of Pluristem’s two lead products, which are currently in clinical trials. She previously headed the University of Haifa Bioinformatics Service Unit, and served as Deloitte’s Incentives Department Life Sciences expert. She completed her post-doctoral training at the prestigious Whitehead Institute for Biomedical Research and at the Technion – Israel Institute of Technology, where she led several multidisciplinary research efforts to address long-standing problems in developmental biology and to develop a single cell transcriptomics assay, which is widely used and cited in the field. She earned her PhD in Biochemistry and BSc (summa cum laude) from the Hebrew University, Jerusalem, Israel. She has received many excellence scholarships and prizes throughout her career and has published numerous works in high-impact journals.
Total Page Views: 5625