Issue:March 2013
FORMULATION DEVELOPMENT - Bioavailability Enhancement Strategies & Opportunities
INTRODUCTION
The therapeutic efficacy of a drug is mainly determined by its bioavailability (BA), which can be simply defined as the rate and extent of absorption of the drug at the target site. Solubility in water, permeability across membranes, resistance to enzyme degradation, as well as stability in different pH ranges are some of the key factors that determine a drug’s BA, though solubility is the top parameter that correlates to the drug’s BA. Generally speaking, a hydrophilic drug has a higher chance of achieving the desired pharmacologic response, when compared to lipophilic ones. Low aqueous solubility might require administration of higher levels of drugs at frequent intervals, which in turn can lead to systemic toxicity for drugs with a narrow therapeutic window.
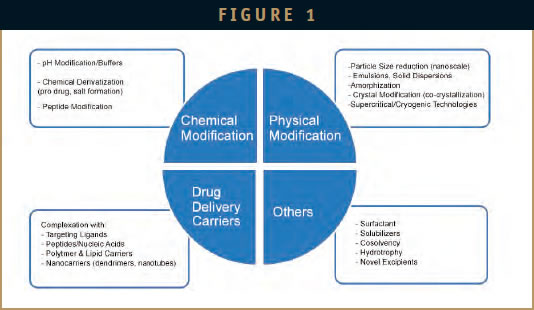
Because a majority of the drugs in development are hydrophobic (estimated at more than 40%), they need to be modified using chemical, physical, and biological methods to improve the aqueous solubility, and hence, BA. Optimization of drug solubility and BA of therapeutics stands as one of the top challenges faced by the pharmaceutical and biotech industry today. A number of new chemical entities (NCEs) and new biological entities (NBEs) in clinical development are facing challenges in late phase of development (Phase II) due to a poor release profile and inability to achieve the desired BA. This can result in high costs, longer development timelines, and delays in regulatory approval. Issues with BA are especially critical for orally administered drugs, as the fraction of drug at the target side is generally much lower in this mode of administration when compared to the intravenous route.
Improvement in BA at the target site is achievable by either delivering the drugs in a form that is more soluble, controlling the release and degradation of the drug (stability), or by deploying active targeting mechanisms for delivery of drug cargo in the tissue/organ of interest. In certain cases, the drugs cannot cross the biological membranes due to the large molecular size, and in such cases, it is important to adopt “trojan horses,” or stealth particles that can cross the barrier by receptor mediated endocytosis. Biological targeting agents, such as cell penetrating peptides, functionalized nanoparticles, monoclonal antibodies, and others are being deployed for active targeting, and this is especially critical for drugs in oncology and neurological applications. A number of drugs act on intracellular targets and require efficient endocytis and permeation to the site of action in a specific organelle in order to exert their pharmacological effects, and poor BA is a key issue for such drugs. Organelle-specific targeting to the mitochondria, endoplasmic reticulum, lysosomes, and so on is a nascent area of research garnering interest from academia and drug developers. By minimizing systemic exposure and maximizing concentration at the target site, smaller doses can be given at less frequent intervals to achieve the same therapeutic effect.
A number of BA-enhancement strategies, such as drug dispersions, selfemulsification, liposomal formulations, size reduction (nanoscale), chemical complexation, and the use of nanocarriers, excipients, and targeting carriers were developed to address issues with specific drugs, and the industry is now striving to develop an integrated range of formulation and delivery strategies that can be cost effectively implemented as a complete toolkit in the drug development process for a wide range of drugs.
Approaches that can mitigate the biological, physical, and chemical barriers for effective delivery of poorly soluble and permeable drugs are being developed by a number of companies working closely with drug developers. Catalent; Merck Millipore; SEPS Pharma; Dow Pharmaceutical Sciences, Inc.; BASF; Aptuit LLC; and Alkermes plc are some of the key technology developers and contract service providers aiding in drug formulation development and manufacture. Enhancement in the BA of a drug cannot only improve patient compliance due to better therapeutic performance and reduced toxicity, but can also aid drug developers in significantly reducing the time and cost of development, while also easing the regulatory approval process.
OPPORTUNITY FOR PRODUCT LINE EXTENSION & PATENTING
Bioavailability boosting technologies not only support product development for new NCEs and NBEs, but also for generic versions of existing drugs and biosimilars. Companies that have developed innovator molecules for specific disease segments can expand their patent and product portfolio by improving the pharmacokinetic profile and dosing parameters of these drugs and also by developing novel formulations that are administrable via different routes. BA and solubility enhancement could also be used as a patenting strategy by companies that are working on generic formulations and biosimilars development. Achieving bioequivalence with alternative routes of administration, such as oral, dermal, mucosal, and pulmonary, with the use of delivery carriers and drug modification techniques could enable biotech and pharmaceutical companies to bring their drugs to the market at a faster pace, while also preventing the failure of drugs in late stages of development.
LIMITATIONS WITH DELIVERY OF PROTEIN-BASED DRUGS: TECHNOLOGY DEVELOPMENTS TO ADDRESS NEEDS
Biologics and other protein-based drugs have emerged as the primary class of drugs for several serious and chronic diseases like cancer, neurology, genetic disorders, and metabolic diseases, in which small molecules have generally not been successful. However, delivery is a challenge with biopharmaceuticals as they are larger and have a more complex molecular structure (when compared to small molecules), which makes it more difficult to administer biopharmaceuticals orally, or via alternative routes.
In spite of the increase in discovery and market approval of NBEs and other protein therapeutics, the issues with poor bioavailability of orally administered proteins and peptide drugs is still a major challenge. Most proteins and peptides undergo rapid degradation and have a very short plasma half-life that makes dosing and delivery difficult. The inflexibility of delivery of biopharmaceuticals has prompted a number of biopharmaceutical, drug delivery, and formulation development companies to explore formulation strategies and drug modification techniques that can improve the efficacy of the drug, and also reduce the dosage required for delivery via oral, pulmonary, and mucosal routes.
With a number of biopharmaceuticals coming off patent, development of novel delivery technologies and formulation techniques can be effectively leveraged by drug developers to carve a niche and gain an edge over others in the competitive biopharmaceuticals industry. The current focus area is on oral and intranasal delivery of complex protein therapeutics, which is still challenging. Some of the key companies working on novel delivery and formulation technologies for protein and peptide drugs are Novozymes Biopharma, UniGene, Aegis Therapeutics, Flamel Technologies, and Aileron Therapeutics. Aegis Therapeutics was awarded several patents for its proprietary technology platforms in 2012. The Intravail technology that enhances transmucosal absorption and protein stabilization excipients (ProTek) technology has also been leveraged by many pharmaceutical companies to develop and commercialize protein-based formulations. Aileron’s Stapled Peptide technology to improve the stability of peptide drugs has garnered interest from the industry, backed by market and pharma majors like Roche, who have entered into a collaborative agreement to leverage the platform. Novozymes Biopharma’s Albufuse (fusion) and Recombumin (conjugation) technologies using recombinant albumin variants to modulate and optimize the halflives of drugs and Tier 1 companies like GlaxoSmithKline and Teva Pharmaceuticals are taking their peptide drugs forward into late clinical development with this technology platform.
A COMPREHENSIVE PORTFOLIO/ESTABLISHMENT OF ALLIANCES
Though there are a number of companies working in specific technologies and strategies for specific drug types, there is a lack of companies that offer integrated formulation and BA boosting techniques that can be offered seamlessly to customers. There is a need to address BA from a more holistic perspective rather than merely addressing it as a solubility issue. Research needs increased focus on combining solubility enhancement along with drug stabilization and targeting techniques to improve BA. Many companies are working toward this by combining individual expertise and developing an integrated solution that can be leveraged by drug developers.
Pharmaceutical and biotech companies are on the lookout for effective manufacturing and formulation technologies for their drug candidates. The number of collaborations between pharma and drug delivery companies, as well as particle engineering organizations, has been on the rise in the past 2 to 3 years. Oncology and central nervous system (CNS) targeted drug delivery are two key areas in which drug solubility enhancement, along with targeted delivery, has gained predominance for existing drug candidates and the development of NCEs. A good example is the partnership between Allergan, Inc. and MAP Pharmaceuticals, Inc. (2011) for development of the orally inhalable formulation LEVADEX for migraine treatment.
While most of the collaborations are research focused and aim to leverage both the companies’ expertise in the area to develop a portfolio of products that can address BA and solubility issues faced by pharma/biotech companies, some of the collaborations have also been in the area of contract manufacturing. The past few years alone witnessed some major deals, such as BASF and Catalent in April 2012, which combines BASF’s expertise in excipient development and formulations with Catalent’s lipid-based delivery systems for BA enhancements. Another example is the more recent collaboration between Hovione and Solvias (December 2012), which is aimed at leveraging both the companies’ strengths to address drug formulation and delivery challenges faced by the industry. This combines Solvia’s solid state chemistry expertise with Hovione’s particle engineering, enhancing solutions they can offer to the pharmaceutical industry.
In addition, Bend Research possesses strong capabilities in formulation development (such as spray-dried dispersion/SDD for low solubility compounds) and has entered into several agreements to capitalize their technology expertise. The collaboration between Bend Research with Dow Chemical (October 2012) and work with other companies like Quotient Bioresearch, Xcelience, Catalent, and Hovione toward integrated solutions for accelerated drug development are some of the most notable recent collaborations. Tier 1 pharma companies and vaccine developers, such as Eli Lilly and Company and PATH, have also leveraged the SDD technology for drug and vaccine formulation development.
BIOAVAILABILITY ENHANCEMENT & DRUG DELIVERY CARRIERS/TARGETING
Liposomes and other lipid-based systems, such as emulsions, have been widely used to improve the BA of hydrophobic drugs for many years and are now being investigated from a different standpoint for targeting. Nano, conjugated, and functionalized liposomes are emerging as advanced solutions for targeted delivery of poorly soluble drugs.
Additionally, cell-penetrating stealth particles, cell targeting homing mechanisms, and environment-sensitive particles are being developed, which is resulting in targeted delivery and, in turn, better BA. Another approach to improve BA is the use of biomimetic particles.
A number of delivery technologies that combine permeability enhancers, protease inhibitors, and solubilizers (BASF) have been developed in the past couple of years. Other methods include particle engineering (including GenSyn Technologies, Inc; Liquidia Technologies; and Enavail), and formation of amorphous dispersions (eg, Veloxis Pharmaceuticals and Catalent) of the drugs, or formation of self-emulsified suspensions to improve oral BA. Because amorphous forms of drugs have better BA (several fold) when compared to crystalline forms (more common), the methods for formation of the same is still a challenge. Acoustic levitation (Argonne National Laboratory) is a new method that has been developed to form amorphous forms of some common APIs to improve BA.
In addition to physical and chemical methods of modification, biological methods, such as conjugation or fusion with targeting ligands/carrier molecules, can improve halflife of a drug or help target the drug to tissue of interest. Oncology is probably the most critical area for targeted delivery. Delivery systems that specifically accumulate in tumors (stimuli responsive, tumor-specific receptors) can improve the efficacy of chemotherapeutics. Delivery to the CNS is also a major challenge due to the presence of the blood brain barrier (BBB).
Improving BA at the target site can significantly reduce drug loads and side effects. Drug delivery systems that can cross primarily include nanoparticles with targeting ligands and CNS-targeting vectors.

Ruplekha Choudhurie is a Senior Research Analyst for Frost & Sullivan’s Technical Insights practice. Her functional expertise includes technical intelligence and competitive benchmarking, in addition to tracking and road-mapping emerging technology trends in the life sciences and biotech sectors that are primed for growth. Ms. Choudhurie also has academic research experience in genetics and microbiology projects. Her knowledge base encompasses genetics/molecular biology, bioprocess engineering, drug discovery, and clinical diagnostics. Prior to joining Frost & Sullivan in 2010, she worked with ABL Biotechnologies and the Children’s Hospital of Philadelphia. She earned her MS in Biotechnology, with a specialization in Biopharmaceuticals/Bioengineering, from the University of Pennsylvania in Philadelphia.
Total Page Views: 3193

















